当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quinone Reduction by Organo-Osmium Half-Sandwich Transfer Hydrogenation Catalysts
Organometallics ( IF 2.5 ) Pub Date : 2021-08-24 , DOI: 10.1021/acs.organomet.1c00358 Elizabeth M. Bolitho 1 , Nathan G. Worby 1 , James P. C. Coverdale 1 , Juliusz A. Wolny 2 , Volker Schünemann 2 , Peter J. Sadler 1
Organometallics ( IF 2.5 ) Pub Date : 2021-08-24 , DOI: 10.1021/acs.organomet.1c00358 Elizabeth M. Bolitho 1 , Nathan G. Worby 1 , James P. C. Coverdale 1 , Juliusz A. Wolny 2 , Volker Schünemann 2 , Peter J. Sadler 1
Affiliation
|
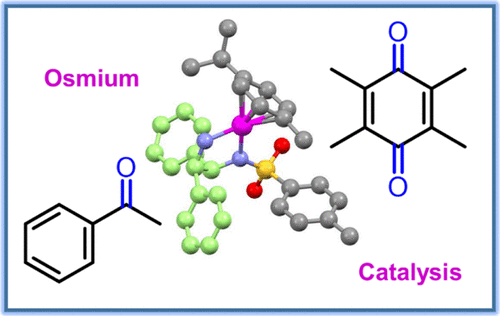
Organo-osmium(II) 16-electron complexes [OsII(η6-arene)(R-PhDPEN)] (where η6-arene = para-cymene or biphenyl) can catalyze the reduction of prochiral ketones to optically pure alcohols in the presence of a hydride source. Such complexes can achieve the conversion of pyruvate to unnatural d-lactate in cancer cells. To improve the catalytic performance of these osmium complexes, we have introduced electron-donor and electron-acceptor substituents (R) into the para (R1) or meta (R2) positions of the chiral R-phenyl-sulfonyl-diphenylethylenediamine (R-PhDPEN) ligands and explored the reduction of quinones, potential biological substrates, which play a major role in cellular electron transfer chains. We show that the series of [OsII(η6-arene)(R-PhDPEN)] derivatives exhibit high turnover frequencies, enantioselectivities (>92%), and conversions (>93%) for the asymmetric transfer hydrogenation (ATH) of acetophenone-derived substrates and reduce duroquinone and menadione to their di-alcohol derivatives. Modeling of the catalysis using density functional theory (DFT) calculations suggests a mechanism involving formic acid deprotonation assisted by the catalyst amine groups, phenyl-duroquinone stacking, hydride transfer to OsII, possible CO2 coordination, and tilting of the η6-arene ring, followed by hydride transfer to the quinone. These findings not only reveal subtle differences between Ru(II) and Os(II) catalysts, but also introduce potential biological applications.
中文翻译:

有机锇半夹心转移加氢催化剂还原醌
有机锇 (II) 16 电子配合物 [Os II (η 6 -芳烃)(R-PhDPEN)](其中 η 6 -芳烃 =对伞花烃或联苯)可以催化前手性酮还原为光学纯醇氢化物源的存在。这种复合物可以在癌细胞中实现丙酮酸向非天然d-乳酸的转化。为了提高这些锇配合物的催化性能,我们在对位(R 1 ) 或间位(R 2) 手性 R-苯基-磺酰基-二苯基乙二胺 (R-PhDPEN) 配体的位置,并探索了在细胞电子转移链中起主要作用的潜在生物底物醌的还原。我们表明 [Os II (η 6 -芳烃)(R-PhDPEN)] 系列衍生物在不对称转移氢化 (ATH) 中表现出高周转频率、对映选择性 (>92%) 和转化率 (>93%)。苯乙酮衍生的底物,并将杜罗醌和甲萘醌还原为它们的二元醇衍生物。使用密度泛函理论 (DFT) 计算的催化模型表明,涉及由催化剂胺基团辅助的甲酸去质子化、苯基-杜罗醌堆积、氢化物转移到 Os II、可能的 CO 的机制2配位和 η 6 -芳烃环倾斜,随后氢化物转移到醌。这些发现不仅揭示了 Ru(II) 和 Os(II) 催化剂之间的细微差别,而且还介绍了潜在的生物学应用。
更新日期:2021-09-13
中文翻译:

有机锇半夹心转移加氢催化剂还原醌
有机锇 (II) 16 电子配合物 [Os II (η 6 -芳烃)(R-PhDPEN)](其中 η 6 -芳烃 =对伞花烃或联苯)可以催化前手性酮还原为光学纯醇氢化物源的存在。这种复合物可以在癌细胞中实现丙酮酸向非天然d-乳酸的转化。为了提高这些锇配合物的催化性能,我们在对位(R 1 ) 或间位(R 2) 手性 R-苯基-磺酰基-二苯基乙二胺 (R-PhDPEN) 配体的位置,并探索了在细胞电子转移链中起主要作用的潜在生物底物醌的还原。我们表明 [Os II (η 6 -芳烃)(R-PhDPEN)] 系列衍生物在不对称转移氢化 (ATH) 中表现出高周转频率、对映选择性 (>92%) 和转化率 (>93%)。苯乙酮衍生的底物,并将杜罗醌和甲萘醌还原为它们的二元醇衍生物。使用密度泛函理论 (DFT) 计算的催化模型表明,涉及由催化剂胺基团辅助的甲酸去质子化、苯基-杜罗醌堆积、氢化物转移到 Os II、可能的 CO 的机制2配位和 η 6 -芳烃环倾斜,随后氢化物转移到醌。这些发现不仅揭示了 Ru(II) 和 Os(II) 催化剂之间的细微差别,而且还介绍了潜在的生物学应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号