当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Non-uniformity of Changes in Drug-Metabolizing Enzymes and Transporters in Liver Cirrhosis: Implications for Drug Dosage Adjustment
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2021-08-24 , DOI: 10.1021/acs.molpharmaceut.1c00462 Eman El-Khateeb 1, 2 , Brahim Achour 1 , Zubida M Al-Majdoub 1 , Jill Barber 1 , Amin Rostami-Hodjegan 1, 3
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2021-08-24 , DOI: 10.1021/acs.molpharmaceut.1c00462 Eman El-Khateeb 1, 2 , Brahim Achour 1 , Zubida M Al-Majdoub 1 , Jill Barber 1 , Amin Rostami-Hodjegan 1, 3
Affiliation
|
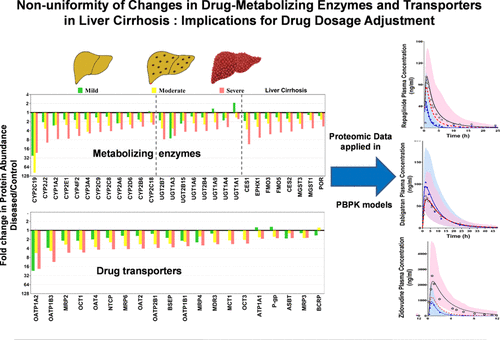
Liver cirrhosis is a chronic disease that affects the liver structure, protein expression, and overall metabolic function. Abundance data for drug-metabolizing enzymes and transporters (DMET) across all stages of disease severity are scarce. Levels of these proteins are crucial for the accurate prediction of drug clearance in hepatically impaired patients using physiologically based pharmacokinetic (PBPK) models, which can be used to guide the selection of more precise dosing. This study aimed to experimentally quantify these proteins in human liver samples and assess how they can impact the predictive performance of the PBPK models. We determined the absolute abundance of 51 DMET proteins in human liver microsomes across the three degrees of cirrhosis severity (n = 32; 6 mild, 13 moderate, and 13 severe), compared to histologically normal controls (n = 14), using QconCAT-based targeted proteomics. The results revealed a significant but non-uniform reduction in the abundance of enzymes and transporters, from control, by 30–50% in mild, 40–70% in moderate, and 50–90% in severe cirrhosis groups. Cancer and/or non-alcoholic fatty liver disease-related cirrhosis showed larger deterioration in levels of CYP3A4, 2C8, 2E1, 1A6, UGT2B4/7, CES1, FMO3/5, EPHX1, MGST1/3, BSEP, and OATP2B1 than the cholestasis set. Drug-specific pathways together with non-uniform changes of abundance across the enzymes and transporters under various degrees of cirrhosis necessitate the use of PBPK models. As case examples, such models for repaglinide, dabigatran, and zidovudine were successful in recovering disease-related alterations in drug exposure. In conclusion, the current study provides the biological rationale behind the absence of a single dose adjustment formula for all drugs in cirrhosis and demonstrates the utility of proteomics-informed PBPK modeling for drug-specific dose adjustment in liver cirrhosis.
中文翻译:

肝硬化药物代谢酶和转运蛋白变化的不均匀性:对药物剂量调整的影响
肝硬化是一种影响肝脏结构、蛋白质表达和整体代谢功能的慢性疾病。疾病严重程度所有阶段的药物代谢酶和转运蛋白 (DMET) 的丰富数据很少。这些蛋白质的水平对于使用基于生理的药代动力学 (PBPK) 模型准确预测肝受损患者的药物清除率至关重要,该模型可用于指导选择更精确的剂量。本研究旨在通过实验量化人类肝脏样本中的这些蛋白质,并评估它们如何影响 PBPK 模型的预测性能。我们确定了三种肝硬化严重程度(n= 32; 6 轻度,13 中度和 13 重度),与组织学正常对照(n= 14),使用基于 QconCAT 的靶向蛋白质组学。结果显示,与对照组相比,酶和转运蛋白的丰度显着但不均匀地减少,轻度肝硬化组减少 30-50%,中度肝硬化组减少 40-70%,重度肝硬化组减少 50-90%。与胆汁淤积相比,癌症和/或非酒精性脂肪性肝病相关肝硬化的 CYP3A4、2C8、2E1、1A6、UGT2B4/7、CES1、FMO3/5、EPHX1、MGST1/3、BSEP 和 OATP2B1 水平的恶化程度更大放。在不同程度的肝硬化下,药物特异性途径以及跨酶和转运蛋白丰度的不均匀变化需要使用 PBPK 模型。例如,瑞格列奈、达比加群和齐多夫定的此类模型成功地恢复了与疾病相关的药物暴露变化。综上所述,
更新日期:2021-09-06
中文翻译:

肝硬化药物代谢酶和转运蛋白变化的不均匀性:对药物剂量调整的影响
肝硬化是一种影响肝脏结构、蛋白质表达和整体代谢功能的慢性疾病。疾病严重程度所有阶段的药物代谢酶和转运蛋白 (DMET) 的丰富数据很少。这些蛋白质的水平对于使用基于生理的药代动力学 (PBPK) 模型准确预测肝受损患者的药物清除率至关重要,该模型可用于指导选择更精确的剂量。本研究旨在通过实验量化人类肝脏样本中的这些蛋白质,并评估它们如何影响 PBPK 模型的预测性能。我们确定了三种肝硬化严重程度(n= 32; 6 轻度,13 中度和 13 重度),与组织学正常对照(n= 14),使用基于 QconCAT 的靶向蛋白质组学。结果显示,与对照组相比,酶和转运蛋白的丰度显着但不均匀地减少,轻度肝硬化组减少 30-50%,中度肝硬化组减少 40-70%,重度肝硬化组减少 50-90%。与胆汁淤积相比,癌症和/或非酒精性脂肪性肝病相关肝硬化的 CYP3A4、2C8、2E1、1A6、UGT2B4/7、CES1、FMO3/5、EPHX1、MGST1/3、BSEP 和 OATP2B1 水平的恶化程度更大放。在不同程度的肝硬化下,药物特异性途径以及跨酶和转运蛋白丰度的不均匀变化需要使用 PBPK 模型。例如,瑞格列奈、达比加群和齐多夫定的此类模型成功地恢复了与疾病相关的药物暴露变化。综上所述,



























 京公网安备 11010802027423号
京公网安备 11010802027423号