当前位置:
X-MOL 学术
›
ACS Appl. Bio Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH and H2S Dual-Responsive Magnetic Metal–Organic Frameworks for Controlling the Release of 5-Fluorouracil
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2021-08-24 , DOI: 10.1021/acsabm.1c00710 Huifang Zhao 1 , Yingjie Zhao 1 , Dahuan Liu 1
ACS Applied Bio Materials ( IF 4.6 ) Pub Date : 2021-08-24 , DOI: 10.1021/acsabm.1c00710 Huifang Zhao 1 , Yingjie Zhao 1 , Dahuan Liu 1
Affiliation
|
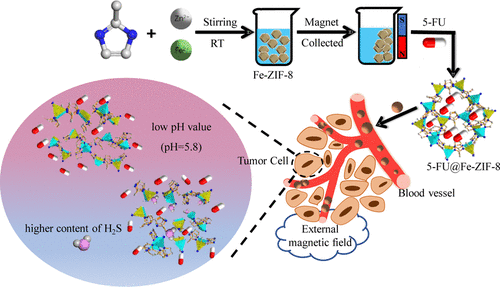
Along with the increasing cancer incidence, developing suitable drug delivery systems (DDSs) is becoming urgent to control drug release and further enhance therapeutic efficiency. Herein, a Fe–Zn bimetallic MOF-derived ferromagnetic nanomaterial was synthesized by a one-step method. The successful preparation of ferromagnetic Fe-ZIF-8 was verified by scanning electron microscopy, powder X-ray diffraction, Brunauer–Emmett–Teller, X-ray photoelectron spectroscopy, and physical property measurement system characterizations. Furthermore, the release behaviors of 5-FU from the ferromagnetic carrier were investigated in a simulative cancer microenvironment of PBS buffer solution (PBS = phosphate-buffered saline, pH = 5.8) and NaHS solution. The vehicle in PBS solution of pH = 5.8 and NaHS solution of 500 μM can rapidly release 5-FU with the cumulative release percentages of 68 and 36%, respectively, within two hundred minutes. The release mechanism in the weak acid environment can be mainly attributed to the decomposition of the Fe-ZIF-8. However, the strong interaction between Zn and Fe atoms in Fe-ZIF-8 and the S atom in H2S plays an important role in the release process in the simulated H2S cancer microenvironment. The investigation of release kinetic models indicates that the 5-FU release in the PBS solutions and NaHS solution of 500 μM can be accurately fitted by a second-degree polynomial model and first-order model, respectively. In addition, the decomposition products, zinc, iron, and 2-MeIM, are endogenous and show low toxicity values [LD50 (Zn) = 0.35 g·kg–1, LD50 (Fe) = 30 g·kg–1, and LD50 (2-MeIM) = 1.4 g·kg–1]. Therefore, the low-toxicity, pH and H2S dual-stimuli-responsive, and ferromagnetic nature make the obtained Fe-ZIF-8 an ideal candidate in the field of bioactive molecule delivery.
中文翻译:

用于控制 5-氟尿嘧啶释放的 pH 和 H2S 双响应磁性金属-有机框架
随着癌症发病率的增加,开发合适的药物递送系统(DDS)以控制药物释放并进一步提高治疗效率变得紧迫。在此,通过一步法合成了一种 Fe-Zn 双金属 MOF 衍生的铁磁纳米材料。通过扫描电子显微镜、粉末 X 射线衍射、Brunauer-Emmett-Teller、X 射线光电子能谱和物理性能测量系统表征验证了铁磁性 Fe-ZIF-8 的成功制备。此外,在 PBS 缓冲溶液(PBS = 磷酸盐缓冲盐水,pH = 5.8)和 NaHS 溶液的模拟癌症微环境中研究了 5-FU 从铁磁载体中的释放行为。载体在 pH = 5 的 PBS 溶液中。8和500 μM的NaHS溶液可在200分钟内快速释放5-FU,累积释放率分别为68%和36%。弱酸环境中的释放机制主要归因于 Fe-ZIF-8 的分解。然而,Fe-ZIF-8 中的 Zn 和 Fe 原子与 H 中的 S 原子之间的强相互作用2 S在模拟的H 2 S癌症微环境中的释放过程中发挥着重要作用。释放动力学模型的研究表明,5-FU在PBS溶液和500 μM NaHS溶液中的释放可以分别通过二阶多项式模型和一阶模型准确拟合。此外,分解产物锌、铁和 2-MeIM 是内源性的,具有低毒性值 [LD 50 (Zn) = 0.35 g·kg –1 , LD 50 (Fe) = 30 g·kg –1 ,和 LD 50 (2-MeIM) = 1.4 g·kg –1 ]。因此,低毒、pH值和H 2S 双刺激响应性和铁磁性使所获得的 Fe-ZIF-8 成为生物活性分子传递领域的理想候选者。
更新日期:2021-09-20
中文翻译:

用于控制 5-氟尿嘧啶释放的 pH 和 H2S 双响应磁性金属-有机框架
随着癌症发病率的增加,开发合适的药物递送系统(DDS)以控制药物释放并进一步提高治疗效率变得紧迫。在此,通过一步法合成了一种 Fe-Zn 双金属 MOF 衍生的铁磁纳米材料。通过扫描电子显微镜、粉末 X 射线衍射、Brunauer-Emmett-Teller、X 射线光电子能谱和物理性能测量系统表征验证了铁磁性 Fe-ZIF-8 的成功制备。此外,在 PBS 缓冲溶液(PBS = 磷酸盐缓冲盐水,pH = 5.8)和 NaHS 溶液的模拟癌症微环境中研究了 5-FU 从铁磁载体中的释放行为。载体在 pH = 5 的 PBS 溶液中。8和500 μM的NaHS溶液可在200分钟内快速释放5-FU,累积释放率分别为68%和36%。弱酸环境中的释放机制主要归因于 Fe-ZIF-8 的分解。然而,Fe-ZIF-8 中的 Zn 和 Fe 原子与 H 中的 S 原子之间的强相互作用2 S在模拟的H 2 S癌症微环境中的释放过程中发挥着重要作用。释放动力学模型的研究表明,5-FU在PBS溶液和500 μM NaHS溶液中的释放可以分别通过二阶多项式模型和一阶模型准确拟合。此外,分解产物锌、铁和 2-MeIM 是内源性的,具有低毒性值 [LD 50 (Zn) = 0.35 g·kg –1 , LD 50 (Fe) = 30 g·kg –1 ,和 LD 50 (2-MeIM) = 1.4 g·kg –1 ]。因此,低毒、pH值和H 2S 双刺激响应性和铁磁性使所获得的 Fe-ZIF-8 成为生物活性分子传递领域的理想候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号