当前位置:
X-MOL 学术
›
Brain Pathol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Upregulation of the pathogenic transcription factor SPI1/PU.1 in tuberous sclerosis complex and focal cortical dysplasia by oxidative stress
Brain Pathology ( IF 5.8 ) Pub Date : 2021-03-30 , DOI: 10.1111/bpa.12949 Till S. Zimmer 1 , Anatoly Korotkov 1 , Susan Zwakenberg 2 , Floor E. Jansen 3 , Fried J. T. Zwartkruis 2 , Nicholas R. Rensing 4 , Michael Wong 4 , Angelika Mühlebner 1, 5 , Erwin A. Vliet 1, 6 , Eleonora Aronica 1, 7 , James D. Mills 1, 8, 9
Brain Pathology ( IF 5.8 ) Pub Date : 2021-03-30 , DOI: 10.1111/bpa.12949 Till S. Zimmer 1 , Anatoly Korotkov 1 , Susan Zwakenberg 2 , Floor E. Jansen 3 , Fried J. T. Zwartkruis 2 , Nicholas R. Rensing 4 , Michael Wong 4 , Angelika Mühlebner 1, 5 , Erwin A. Vliet 1, 6 , Eleonora Aronica 1, 7 , James D. Mills 1, 8, 9
Affiliation
|
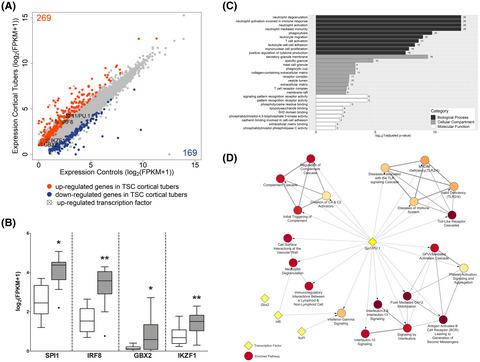
Tuberous sclerosis complex (TSC) is a congenital disorder characterized by cortical malformations and concomitant epilepsy caused by loss-of-function mutations in the mTOR suppressors TSC1 or TSC2. While the underlying molecular changes caused by mTOR activation in TSC have previously been investigated, the drivers of these transcriptional change have not been fully elucidated. A better understanding of the perturbed transcriptional regulation could lead to the identification of novel pathways for therapeutic intervention not only in TSC, but other genetic epilepsies in which mTOR activation plays a key role, such as focal cortical dysplasia 2b (FCD). Here, we analyzed RNA sequencing data from cortical tubers and a tsc2−/− zebrafish. We identified differential expression of the transcription factors (TFs) SPI1/PU.1, IRF8, GBX2, and IKZF1 of which SPI1/PU.1 and IRF8 targets were enriched among the differentially expressed genes. Furthermore, for SPI1/PU.1 these findings were conserved in TSC zebrafish model. Next, we confirmed overexpression of SPI1/PU.1 on the RNA and protein level in a separate cohort of surgically resected TSC tubers and FCD tissue, in fetal TSC tissue, and a Tsc1GFAP−/− mouse model of TSC. Subsequently, we validated the expression of SPI1/PU.1 in dysmorphic cells with mTOR activation in TSC tubers. In fetal TSC, we detected SPI1/PU.1 expression prenatally and elevated RNA Spi1 expression in Tsc1GFAP−/− mice before the development of seizures. Finally, in vitro, we identified that in astrocytes and neurons SPI1 transcription was driven by H2O2-induced oxidative stress, independent of mTOR. We identified SPI1/PU.1 as a novel TF involved in the pro-inflammatory gene expression of malformed cells in TSC and FCD 2b. This transcriptional program is activated in response to oxidative stress and already present prenatally. Importantly, SPI1/PU.1 protein appears to be strictly limited to malformed cells, as we did not find SPI1/PU.1 protein expression in mice nor in our in vitro models.
中文翻译:

氧化应激对结节性硬化症和局灶性皮质发育不良中致病性转录因子 SPI1/PU.1 的上调
结节性硬化症 (TSC) 是一种先天性疾病,其特征是皮质畸形和伴随的癫痫症,由 mTOR 抑制基因TSC1或TSC2的功能丧失突变引起。虽然之前已经研究了 TSC 中 mTOR 激活引起的潜在分子变化,但尚未完全阐明这些转录变化的驱动因素。更好地理解扰动的转录调控可以导致确定治疗干预的新途径,不仅在 TSC 中,而且在 mTOR 激活起关键作用的其他遗传性癫痫中,例如局灶性皮质发育不良 2b (FCD)。在这里,我们分析了来自皮质块茎和 tsc2 -/- 的RNA 测序数据斑马鱼。我们确定了转录因子 (TF) SPI1/PU.1、IRF8、GBX2 和 IKZF1 的差异表达,其中 SPI1/PU.1 和 IRF8 靶标在差异表达基因中富集。此外,对于 SPI1/PU.1,这些发现在 TSC 斑马鱼模型中是保守的。接下来,我们证实了在手术切除的 TSC 块茎和 FCD 组织的单独队列、胎儿 TSC 组织和Tsc1 GFAP-/- TSC 小鼠模型中,SPI1/PU.1 在 RNA 和蛋白质水平上的过度表达。随后,我们验证了 SPI1/PU.1 在 TSC 块茎中 mTOR 激活的畸形细胞中的表达。在胎儿 TSC 中,我们在产前检测到 SPI1/PU.1 表达,并在癫痫发作前Tsc1 GFAP-/-小鼠中检测到 RNA Spi1 表达升高。最后,在体外,我们发现在星形胶质细胞和神经元中,SPI1转录由 H 2 O 2诱导的氧化应激驱动,与 mTOR 无关。我们将 SPI1/PU.1 鉴定为一种新型 TF,其参与 TSC 和 FCD 2b 中畸形细胞的促炎基因表达。该转录程序响应氧化应激而被激活,并且在出生前就已经存在。重要的是,SPI1/PU.1 蛋白似乎严格限于畸形细胞,因为我们在小鼠和体外模型中都没有发现 SPI1/PU.1 蛋白表达。
更新日期:2021-03-30
中文翻译:

氧化应激对结节性硬化症和局灶性皮质发育不良中致病性转录因子 SPI1/PU.1 的上调
结节性硬化症 (TSC) 是一种先天性疾病,其特征是皮质畸形和伴随的癫痫症,由 mTOR 抑制基因TSC1或TSC2的功能丧失突变引起。虽然之前已经研究了 TSC 中 mTOR 激活引起的潜在分子变化,但尚未完全阐明这些转录变化的驱动因素。更好地理解扰动的转录调控可以导致确定治疗干预的新途径,不仅在 TSC 中,而且在 mTOR 激活起关键作用的其他遗传性癫痫中,例如局灶性皮质发育不良 2b (FCD)。在这里,我们分析了来自皮质块茎和 tsc2 -/- 的RNA 测序数据斑马鱼。我们确定了转录因子 (TF) SPI1/PU.1、IRF8、GBX2 和 IKZF1 的差异表达,其中 SPI1/PU.1 和 IRF8 靶标在差异表达基因中富集。此外,对于 SPI1/PU.1,这些发现在 TSC 斑马鱼模型中是保守的。接下来,我们证实了在手术切除的 TSC 块茎和 FCD 组织的单独队列、胎儿 TSC 组织和Tsc1 GFAP-/- TSC 小鼠模型中,SPI1/PU.1 在 RNA 和蛋白质水平上的过度表达。随后,我们验证了 SPI1/PU.1 在 TSC 块茎中 mTOR 激活的畸形细胞中的表达。在胎儿 TSC 中,我们在产前检测到 SPI1/PU.1 表达,并在癫痫发作前Tsc1 GFAP-/-小鼠中检测到 RNA Spi1 表达升高。最后,在体外,我们发现在星形胶质细胞和神经元中,SPI1转录由 H 2 O 2诱导的氧化应激驱动,与 mTOR 无关。我们将 SPI1/PU.1 鉴定为一种新型 TF,其参与 TSC 和 FCD 2b 中畸形细胞的促炎基因表达。该转录程序响应氧化应激而被激活,并且在出生前就已经存在。重要的是,SPI1/PU.1 蛋白似乎严格限于畸形细胞,因为我们在小鼠和体外模型中都没有发现 SPI1/PU.1 蛋白表达。











































 京公网安备 11010802027423号
京公网安备 11010802027423号