当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Access to α-Aminosilanes Enabled by Visible-Light-Mediated Multicomponent Radical Cross-Coupling
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-08-25 , DOI: 10.1002/anie.202109252 Xiaoye Yu 1 , Constantin G Daniliuc 1 , Fatmah Ali Alasmary 2 , Armido Studer 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-08-25 , DOI: 10.1002/anie.202109252 Xiaoye Yu 1 , Constantin G Daniliuc 1 , Fatmah Ali Alasmary 2 , Armido Studer 1, 2
Affiliation
|
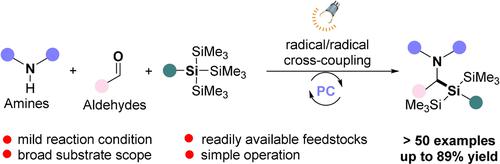
α-Aminosilanes are an important class of organic compounds that show biological activity. In this communication, a new approach to α-aminosilanes that utilizes photoredox catalysis to enable three-component coupling of organo(tristrimethylsilyl)silanes with feedstock alkylamines and aldehydes is presented. A wide range of highly functionalized α-aminosilanes can be obtained in good yields under mild conditions. Both primary amines and secondary amines are compatible with this transformation. Moreover, optically pure α-aminosilanes are accessible by using chiral amines. Mechanistic studies indicate that reactions proceed through radical/radical cross-coupling of silyl radicals with α-amino alkyl radicals.
中文翻译:

通过可见光介导的多组分自由基交叉偶联直接获得α-氨基硅烷
α-氨基硅烷是一类具有生物活性的重要有机化合物。在本次交流中,提出了一种α-氨基硅烷的新方法,该方法利用光氧化还原催化使有机(三三甲基硅基)硅烷与原料烷基胺和醛进行三组分偶联。在温和条件下可以以良好的产率获得各种高度官能化的 α-氨基硅烷。伯胺和仲胺都适合这种转变。此外,通过使用手性胺可以获得光学纯的α-氨基硅烷。机理研究表明反应是通过甲硅烷基与α-氨基烷基的自由基/自由基交叉偶联进行的。
更新日期:2021-10-12
中文翻译:

通过可见光介导的多组分自由基交叉偶联直接获得α-氨基硅烷
α-氨基硅烷是一类具有生物活性的重要有机化合物。在本次交流中,提出了一种α-氨基硅烷的新方法,该方法利用光氧化还原催化使有机(三三甲基硅基)硅烷与原料烷基胺和醛进行三组分偶联。在温和条件下可以以良好的产率获得各种高度官能化的 α-氨基硅烷。伯胺和仲胺都适合这种转变。此外,通过使用手性胺可以获得光学纯的α-氨基硅烷。机理研究表明反应是通过甲硅烷基与α-氨基烷基的自由基/自由基交叉偶联进行的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号