当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preclinical Evaluation of [64Cu]NOTA-CP01 as a PET Imaging Agent for Metastatic Esophageal Squamous Cell Carcinoma
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2021-08-23 , DOI: 10.1021/acs.molpharmaceut.1c00600 Tukang Peng 1 , Xiaohui Wang 1 , Zhijun Li 1 , Lei Bi 1 , Jiebing Gao 2 , Min Yang 1 , Yuwei Wang 3, 4 , Xiaojun Yao 3 , Hong Shan 1, 5 , Hongjun Jin 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2021-08-23 , DOI: 10.1021/acs.molpharmaceut.1c00600 Tukang Peng 1 , Xiaohui Wang 1 , Zhijun Li 1 , Lei Bi 1 , Jiebing Gao 2 , Min Yang 1 , Yuwei Wang 3, 4 , Xiaojun Yao 3 , Hong Shan 1, 5 , Hongjun Jin 1
Affiliation
|
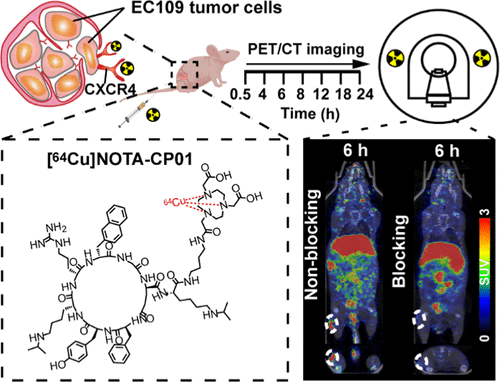
Targeting metastatic esophageal squamous cell carcinoma (ESCC) has been a challenge in clinical practice. Emerging evidence demonstrates that C-X-C chemokine receptor 4 (CXCR4) highly expresses in ESCC and plays a pivotal role in the process of tumor metastasis. We developed a copper-64 (t1/2 = 12.7 h, 19% beta+) labeling route of NOTA-CP01 derived from LY2510924, a cyclopeptide-based CXCR4 potent antagonist, in an attempt to noninvasively visualize CXCR4 expression in metastatic ESCC. Precursor NOTA-CP01 was designed by modifying the C-terminus of LY2510925 with bis-t-butyl NOTA via a butane-1,4-diamine linker. The radiolabeling process was finished within 15 min with high radiochemical yield (>95%), radiochemical purity (>99%), and specific activity (10.5–21 GBq/μmol) (non-decay-corrected). The in vitro solubility and stability tests revealed that [64Cu]NOTA-CP01 had a high water solubility (log P = −3.44 ± 0.12, n = 5) and high stability in saline and fetal bovine serum. [64Cu]NOTA-CP01 exhibited CXCR4-specific binding with a nanomolar affinity (IC50 = 1.61 ± 0.96 nM, Kd = 0.272 ± 0.14 nM) similar to that of the parental LY2510924. The in vitro cell uptake assay indicated that the [64Cu]NOTA-CP01-selective accumulation in EC109 cells was CXCR4-specific. Molecular docking of the CXCR4/NOTA-CP01 complex suggested that the Lys, Arg, and NOTA of this ligand have a strong polar interaction with the key residues of CXCR4, which explains the tight affinity of [64Cu]NOTA-CP01 for CXCR4. To test the target engagement in vivo, prolonged-time positron emission computed tomography (PET) imaging was performed at 0.5, 4, 6, 8, 12, 16, and 24 h postinjection of [64Cu]NOTA-CP01 to the EC109 tumor-bearing mice. The EC109 tumors were most visible with high contrast to the contralateral background at 6 h postinjection. The tracer revealed receptor-specific tumor accumulation, which was illustrated by effective blocking via coinjection with a blocking dose of LY2510924. Quantification analysis of the prolonged-time images showed that there was obvious radioactivity accumulation in the tumor (1.27 ± 0.19%ID/g) with the best tumor-to-blood ratio (4.79 ± 0.06) and tumor-to-muscle ratio (15.44 ± 2.94) at 6 h postinjection of the probe. The immunofluorescence and immunohistochemistry confirmed the positive expression of CXCR4 in the EC109 tumor and ESCC and metastatic lymph nodes of patients, respectively. We concluded that [64Cu]NOTA-CP01 possessed a very high target engagement for CXCR4-positive ESCC and could be a potential candidate in the clinical detection of metastatic ESCC.
中文翻译:

[64Cu]NOTA-CP01作为PET显像剂治疗转移性食管鳞状细胞癌的临床前评价
靶向转移性食管鳞状细胞癌(ESCC)一直是临床实践中的挑战。新出现的证据表明,CXC 趋化因子受体 4 (CXCR4) 在 ESCC 中高表达,在肿瘤转移过程中起关键作用。我们开发了一种源自 LY2510924(一种基于环肽的 CXCR4 强效拮抗剂)的 NOTA-CP01 的铜 64 ( t 1/2 = 12.7 h, 19% β + ) 标记途径,试图无创地观察转移性 ESCC 中的 CXCR4 表达。前体 NOTA-CP01 是通过修改 LY2510925 的 C 端与 bis- t-丁基 NOTA 通过丁烷-1,4-二胺接头。放射性标记过程在 15 分钟内完成,具有高放射化学产率 (>95%)、放射化学纯度 (>99%) 和比活度 (10.5–21 GBq/μmol)(未经过衰减校正)。体外溶解度和稳定性测试表明,[ 64 Cu ]NOTA-CP01 具有高水溶性(log P = -3.44 ± 0.12,n = 5),在盐水和胎牛血清中具有高稳定性。[ 64 Cu]NOTA-CP01 表现出与亲本 LY2510924 相似的CXCR4 特异性结合,具有纳摩尔亲和力(IC 50 = 1.61 ± 0.96 nM,K d = 0.272 ± 0.14 nM)。体外的细胞摄取试验表明,[ 64 Cu]NOTA-CP01 在 EC109 细胞中的选择性积累是 CXCR4 特异性的。CXCR4/NOTA-CP01 复合物的分子对接表明该配体的 Lys、Arg 和 NOTA 与 CXCR4 的关键残基具有强烈的极性相互作用,这解释了 [ 64 Cu]NOTA-CP01 对 CXCR4 的紧密亲和力。为了测试体内的目标接合,在注射 [ 64 ] 后 0.5、4、6、8、12、16 和 24 小时进行长时间正电子发射计算机断层扫描 (PET) 成像Cu]NOTA-CP01 对 EC109 荷瘤小鼠。EC109 肿瘤在注射后 6 小时最明显,与对侧背景具有高对比度。示踪剂显示受体特异性肿瘤积聚,通过与阻断剂量 LY2510924 共同注射的有效阻断说明了这一点。长时间图像的定量分析显示,肿瘤内有明显的放射性积累(1.27±0.19%ID/g),肿瘤与血液的比率(4.79±0.06)和肿瘤与肌肉的比率(15.44)最佳。 ± 2.94) 在探针注射后 6 小时。免疫荧光和免疫组化分别证实了 CXCR4 在 EC109 肿瘤和患者 ESCC 和转移淋巴结中的阳性表达。我们得出结论 [ 64Cu]NOTA-CP01 对 CXCR4 阳性 ESCC 具有非常高的靶标参与度,可能是转移性 ESCC 临床检测的潜在候选者。
更新日期:2021-09-06
中文翻译:

[64Cu]NOTA-CP01作为PET显像剂治疗转移性食管鳞状细胞癌的临床前评价
靶向转移性食管鳞状细胞癌(ESCC)一直是临床实践中的挑战。新出现的证据表明,CXC 趋化因子受体 4 (CXCR4) 在 ESCC 中高表达,在肿瘤转移过程中起关键作用。我们开发了一种源自 LY2510924(一种基于环肽的 CXCR4 强效拮抗剂)的 NOTA-CP01 的铜 64 ( t 1/2 = 12.7 h, 19% β + ) 标记途径,试图无创地观察转移性 ESCC 中的 CXCR4 表达。前体 NOTA-CP01 是通过修改 LY2510925 的 C 端与 bis- t-丁基 NOTA 通过丁烷-1,4-二胺接头。放射性标记过程在 15 分钟内完成,具有高放射化学产率 (>95%)、放射化学纯度 (>99%) 和比活度 (10.5–21 GBq/μmol)(未经过衰减校正)。体外溶解度和稳定性测试表明,[ 64 Cu ]NOTA-CP01 具有高水溶性(log P = -3.44 ± 0.12,n = 5),在盐水和胎牛血清中具有高稳定性。[ 64 Cu]NOTA-CP01 表现出与亲本 LY2510924 相似的CXCR4 特异性结合,具有纳摩尔亲和力(IC 50 = 1.61 ± 0.96 nM,K d = 0.272 ± 0.14 nM)。体外的细胞摄取试验表明,[ 64 Cu]NOTA-CP01 在 EC109 细胞中的选择性积累是 CXCR4 特异性的。CXCR4/NOTA-CP01 复合物的分子对接表明该配体的 Lys、Arg 和 NOTA 与 CXCR4 的关键残基具有强烈的极性相互作用,这解释了 [ 64 Cu]NOTA-CP01 对 CXCR4 的紧密亲和力。为了测试体内的目标接合,在注射 [ 64 ] 后 0.5、4、6、8、12、16 和 24 小时进行长时间正电子发射计算机断层扫描 (PET) 成像Cu]NOTA-CP01 对 EC109 荷瘤小鼠。EC109 肿瘤在注射后 6 小时最明显,与对侧背景具有高对比度。示踪剂显示受体特异性肿瘤积聚,通过与阻断剂量 LY2510924 共同注射的有效阻断说明了这一点。长时间图像的定量分析显示,肿瘤内有明显的放射性积累(1.27±0.19%ID/g),肿瘤与血液的比率(4.79±0.06)和肿瘤与肌肉的比率(15.44)最佳。 ± 2.94) 在探针注射后 6 小时。免疫荧光和免疫组化分别证实了 CXCR4 在 EC109 肿瘤和患者 ESCC 和转移淋巴结中的阳性表达。我们得出结论 [ 64Cu]NOTA-CP01 对 CXCR4 阳性 ESCC 具有非常高的靶标参与度,可能是转移性 ESCC 临床检测的潜在候选者。











































 京公网安备 11010802027423号
京公网安备 11010802027423号