Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cationization of neutral small molecules by site-specific carboxylation of 10-phenyl-10H-phenothiazine in laser desorption/ionization
Analyst ( IF 3.6 ) Pub Date : 2021-08-03 , DOI: 10.1039/d1an01111a Chongqing Ma 1 , Tianrong Yu 1 , Yue Liu 1 , Rui Shi 1 , Rui Lv 1 , Ruochen Guo 1 , Qinghua Cao 1 , Gaole Dai 1 , Yu Zhao 1 , Jian Liu 1
Analyst ( IF 3.6 ) Pub Date : 2021-08-03 , DOI: 10.1039/d1an01111a Chongqing Ma 1 , Tianrong Yu 1 , Yue Liu 1 , Rui Shi 1 , Rui Lv 1 , Ruochen Guo 1 , Qinghua Cao 1 , Gaole Dai 1 , Yu Zhao 1 , Jian Liu 1
Affiliation
|
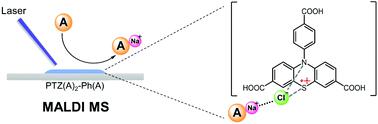
It is a pre-requisite to ionize analyte molecules efficiently for detection by laser desorption/ionization mass spectrometry. Here, we report a conceptual demonstration of cationizing neutral small molecules which are typically difficult to be ionized with the traditional organic matrices due to their low proton/cation affinity values. Our strategy features generating radical cations from site-specifically carboxylated 10-(4-carboxyphenyl)-10H-phenothiazine-3,7-dicarboxylic acid (PTZ(A)2-Ph(A)) with a laser, and anchoring the chlorine ion from NaCl through covalent bond-like bridging interactions with the N/S atoms in the heterocyclic structure. This “Maverick” design allows a dramatic change of the energy landscape of analyte sodiation with an enhanced efficiency. We have synthesized two families of compounds based on the model structures of phenothiazine (PTZ) and phenoxazine (PXZ) and their carboxylated derivatives, and performed comparison between them or against the traditional organic matrices in a systematic format. We have demonstrated that PTZ(A)2-Ph(A) is outstanding as a novel MALDI matrix for the detection of oligosaccharides and amino acids, with an ultra-clean background baseline and high signal-to-noise ratios (up to dozens of times better than the traditional matrices). This work provides a new method for the cationization of neutral small molecules in a distinct mechanism, inspiring the development of next-generation matrices for sensitive detection of hard-to-be-ionized molecules by MALDI MS.
中文翻译:

在激光解吸/电离中通过 10-苯基-10H-吩噻嗪的位点特异性羧化使中性小分子阳离子化
有效地电离分析物分子以通过激光解吸/电离质谱法进行检测是先决条件。在这里,我们报告了阳离子化中性小分子的概念性演示,由于质子/阳离子亲和力值低,这些小分子通常难以被传统有机基质电离。我们的策略特点是从位点特异性羧化的 10-(4-carboxyphenyl)-10 H-吩噻嗪-3,7-二羧酸 (PTZ(A) 2-Ph(A)),并通过与杂环结构中的 N/S 原子的共价键式桥接相互作用将 NaCl 中的氯离子锚定。这种“特立独行”的设计允许以更高的效率显着改变分析物钠化的能量格局。我们基于吩噻嗪 (PTZ) 和吩恶嗪 (PXZ) 及其羧化衍生物的模型结构合成了两大族化合物,并以系统的形式进行了它们之间的比较或与传统有机基质的比较。我们已经证明 PTZ(A) 2-Ph(A) 作为一种用于检测寡糖和氨基酸的新型 MALDI 基质非常出色,具有超干净的背景基线和高信噪比(比传统基质好几十倍)。这项工作为中性小分子以独特的机制阳离子化提供了一种新方法,启发了下一代基质的开发,用于通过 MALDI MS 灵敏检测难以电离的分子。
更新日期:2021-08-24
中文翻译:

在激光解吸/电离中通过 10-苯基-10H-吩噻嗪的位点特异性羧化使中性小分子阳离子化
有效地电离分析物分子以通过激光解吸/电离质谱法进行检测是先决条件。在这里,我们报告了阳离子化中性小分子的概念性演示,由于质子/阳离子亲和力值低,这些小分子通常难以被传统有机基质电离。我们的策略特点是从位点特异性羧化的 10-(4-carboxyphenyl)-10 H-吩噻嗪-3,7-二羧酸 (PTZ(A) 2-Ph(A)),并通过与杂环结构中的 N/S 原子的共价键式桥接相互作用将 NaCl 中的氯离子锚定。这种“特立独行”的设计允许以更高的效率显着改变分析物钠化的能量格局。我们基于吩噻嗪 (PTZ) 和吩恶嗪 (PXZ) 及其羧化衍生物的模型结构合成了两大族化合物,并以系统的形式进行了它们之间的比较或与传统有机基质的比较。我们已经证明 PTZ(A) 2-Ph(A) 作为一种用于检测寡糖和氨基酸的新型 MALDI 基质非常出色,具有超干净的背景基线和高信噪比(比传统基质好几十倍)。这项工作为中性小分子以独特的机制阳离子化提供了一种新方法,启发了下一代基质的开发,用于通过 MALDI MS 灵敏检测难以电离的分子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号