当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereospecific Synthesis of Conjugated Dienes by Carbenoid Eliminative Cross-Coupling Using Lithiated Allylic Carbamates
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2021-08-23 , DOI: 10.1002/ejoc.202101011 Subhash D. Tanpure 1 , Paul R. Blakemore 2
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2021-08-23 , DOI: 10.1002/ejoc.202101011 Subhash D. Tanpure 1 , Paul R. Blakemore 2
Affiliation
|
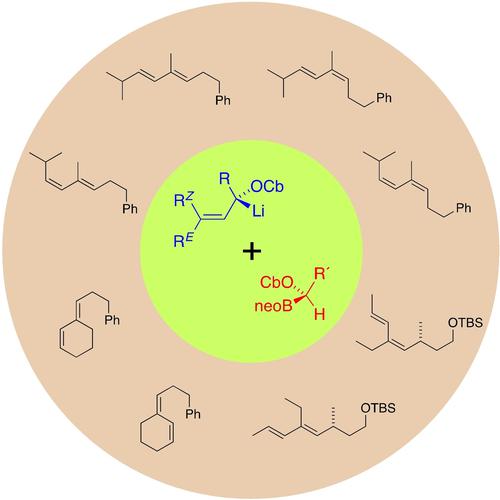
In control! Eliminative cross-coupling between enantioenriched lithiated allylic carbamates and enantioenriched α-carbamoyloxyalkyl boronates provides for a stereospecific synthesis of 1,2,4-trisubstituted 1,3-butadienes. Any geometrical isomer of the conjugated diene product is available by an appropriate combination of stereochemical configurations within the carbenoid substrates and the type of elimination mechanism triggered (syn or anti).

中文翻译:

使用锂化烯丙基氨基甲酸酯通过类卡宾消除交叉偶联立体有择合成共轭二烯
掌控之中!对映体富集的锂化烯丙基氨基甲酸酯和对映体富集的α-氨基甲酰氧基烷基硼酸酯之间的消除交叉偶联提供了 1,2,4-三取代的 1,3-丁二烯的立体有择合成。共轭二烯产物的任何几何异构体都可以通过类卡宾底物中的立体化学构型和触发的消除机制类型(顺式或反式)的适当组合获得。

更新日期:2021-09-15

中文翻译:

使用锂化烯丙基氨基甲酸酯通过类卡宾消除交叉偶联立体有择合成共轭二烯
掌控之中!对映体富集的锂化烯丙基氨基甲酸酯和对映体富集的α-氨基甲酰氧基烷基硼酸酯之间的消除交叉偶联提供了 1,2,4-三取代的 1,3-丁二烯的立体有择合成。共轭二烯产物的任何几何异构体都可以通过类卡宾底物中的立体化学构型和触发的消除机制类型(顺式或反式)的适当组合获得。












































 京公网安备 11010802027423号
京公网安备 11010802027423号