当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Influence of the Oxygen Reduction Reaction (ORR) on Pt Oxide Electrochemistry
ChemElectroChem ( IF 3.5 ) Pub Date : 2021-08-24 , DOI: 10.1002/celc.202100710 Oliver Rodríguez 1 , Guy DENUAULT 2
ChemElectroChem ( IF 3.5 ) Pub Date : 2021-08-24 , DOI: 10.1002/celc.202100710 Oliver Rodríguez 1 , Guy DENUAULT 2
Affiliation
|
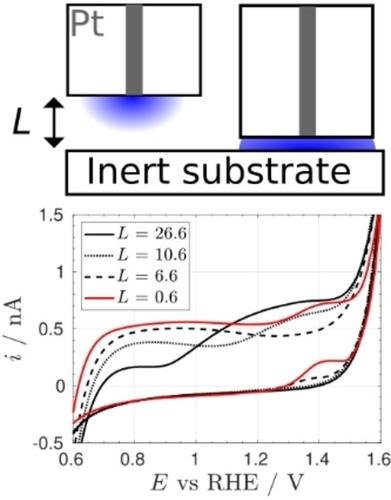
In this study, we employed microelectrodes and scanning electrochemical microscopy (SECM) to investigate the role of molecular oxygen and local pH changes on the electrochemistry of Pt oxide. We show that in acidic media and alkaline conditions, the impact of O2 is negligible, while in unbuffered neutral media, O2 strongly affects the formation of Pt oxides. Experiments carried under hindered diffusion reveal that this is due to a high local pH arising from the oxygen reduction reaction. This is evidenced by the appearance, at very positive potentials, of a diffusion controlled wave consistent with the oxidation of OH−. The ORR produces a sufficiently alkaline environment near the electrode to promote the formation of oxide at much more negative potentials than anticipated from the bulk pH. As a result, the onset of oxide formation overlaps the onset of oxygen reduction and it is impossible to obtain a Pt surface free from oxide at potentials positive of the onset of the ORR. Thus, prior exposure of the Pt surface to dissolved oxygen does not leave irreversibly adsorbed oxygen species as previously reported by our group; instead, the ORR induces a coverage of oxide at much lower potentials than determined by the bulk pH.
中文翻译:

氧还原反应(ORR)对氧化铂电化学的影响
在这项研究中,我们使用微电极和扫描电化学显微镜 (SECM) 来研究分子氧和局部 pH 值变化对 Pt 氧化物电化学的作用。我们表明,在酸性介质和碱性条件下,O 2的影响可以忽略不计,而在无缓冲的中性介质中,O 2强烈影响 Pt 氧化物的形成。在受阻扩散下进行的实验表明,这是由于氧还原反应引起的高局部 pH 值。这可以通过在非常正的电位下出现与 OH -氧化一致的扩散控制波来证明。. ORR 在电极附近产生足够碱性的环境,以促进在比本体 pH 值预期低得多的负电位下形成氧化物。因此,氧化物形成的开始与氧还原的开始重叠,并且不可能在 ORR 开始的正电位下获得没有氧化物的 Pt 表面。因此,Pt 表面预先暴露于溶解氧不会像我们小组先前报道的那样留下不可逆转的吸附氧物质。相反,ORR 会在比整体 pH 值低得多的电位下诱导氧化物覆盖。
更新日期:2021-09-13
中文翻译:

氧还原反应(ORR)对氧化铂电化学的影响
在这项研究中,我们使用微电极和扫描电化学显微镜 (SECM) 来研究分子氧和局部 pH 值变化对 Pt 氧化物电化学的作用。我们表明,在酸性介质和碱性条件下,O 2的影响可以忽略不计,而在无缓冲的中性介质中,O 2强烈影响 Pt 氧化物的形成。在受阻扩散下进行的实验表明,这是由于氧还原反应引起的高局部 pH 值。这可以通过在非常正的电位下出现与 OH -氧化一致的扩散控制波来证明。. ORR 在电极附近产生足够碱性的环境,以促进在比本体 pH 值预期低得多的负电位下形成氧化物。因此,氧化物形成的开始与氧还原的开始重叠,并且不可能在 ORR 开始的正电位下获得没有氧化物的 Pt 表面。因此,Pt 表面预先暴露于溶解氧不会像我们小组先前报道的那样留下不可逆转的吸附氧物质。相反,ORR 会在比整体 pH 值低得多的电位下诱导氧化物覆盖。











































 京公网安备 11010802027423号
京公网安备 11010802027423号