当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electronic Modulation Caused by Interfacial Ni-O-M (M=Ru, Ir, Pd) Bonding for Accelerating Hydrogen Evolution Kinetics
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-08-23 , DOI: 10.1002/anie.202110374 Liming Deng, Feng Hu, Mingyue Ma, Shao-Chu Huang, Yixing Xiong, Han-Yi Chen, Linlin Li, Shengjie Peng
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2021-08-23 , DOI: 10.1002/anie.202110374 Liming Deng, Feng Hu, Mingyue Ma, Shao-Chu Huang, Yixing Xiong, Han-Yi Chen, Linlin Li, Shengjie Peng
|
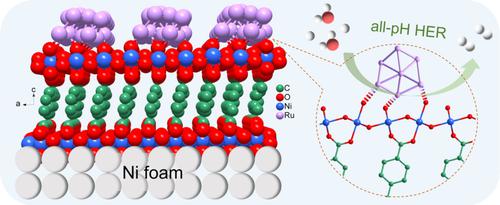
Designing definite metal-support interfacial bond is an effective strategy for optimizing the intrinsic activity of noble metals, but rather challenging. Herein, a series of quantum-sized metal nanoparticles (NPs) anchored on nickel metal–organic framework nanohybrids (M@Ni-MOF, M=Ru, Ir, Pd) are rationally developed through a spontaneous redox strategy. The metal-oxygen bonds between the NPs and Ni-MOF guarantee structural stability and sufficient exposure of the surface active sites. More importantly, such precise interfacial feature can effectively modulate the electronic structure of hybrids through the charge transfer of the formed Ni-O-M bridge and then improves the reaction kinetics. As a result, the representative Ru@Ni-MOF exhibits excellent hydrogen evolution reaction (HER) activity at all pH values, even superior to commercial Pt/C and recent noble-metal catalysts. Theoretical calculations deepen the mechanism understanding of the superior HER performance of Ru@Ni-MOF through the optimized adsorption free energies of water and hydrogen due to the interfacial-bond-induced electron redistribution.
中文翻译:

界面 Ni-OM (M=Ru, Ir, Pd) 键合引起的电子调制加速氢演化动力学
设计明确的金属-载体界面键是优化贵金属内在活性的有效策略,但具有挑战性。在此,通过自发氧化还原策略合理开发了一系列锚定在镍金属-有机骨架纳米杂化物(M@Ni-MOF,M=Ru,Ir,Pd)上的量子尺寸金属纳米颗粒(NPs)。NPs 和 Ni-MOF 之间的金属-氧键保证了结构稳定性和表面活性位点的充分暴露。更重要的是,这种精确的界面特征可以通过形成的 Ni-OM 桥的电荷转移有效地调节杂化物的电子结构,从而改善反应动力学。因此,代表性的 Ru@Ni-MOF 在所有 pH 值下都表现出优异的析氢反应(HER)活性,甚至优于商业 Pt/C 和最近的贵金属催化剂。理论计算通过界面键诱导电子重新分布优化水和氢的吸附自由能,加深了对 Ru@Ni-MOF 优异 HER 性能的机理理解。
更新日期:2021-09-27
中文翻译:

界面 Ni-OM (M=Ru, Ir, Pd) 键合引起的电子调制加速氢演化动力学
设计明确的金属-载体界面键是优化贵金属内在活性的有效策略,但具有挑战性。在此,通过自发氧化还原策略合理开发了一系列锚定在镍金属-有机骨架纳米杂化物(M@Ni-MOF,M=Ru,Ir,Pd)上的量子尺寸金属纳米颗粒(NPs)。NPs 和 Ni-MOF 之间的金属-氧键保证了结构稳定性和表面活性位点的充分暴露。更重要的是,这种精确的界面特征可以通过形成的 Ni-OM 桥的电荷转移有效地调节杂化物的电子结构,从而改善反应动力学。因此,代表性的 Ru@Ni-MOF 在所有 pH 值下都表现出优异的析氢反应(HER)活性,甚至优于商业 Pt/C 和最近的贵金属催化剂。理论计算通过界面键诱导电子重新分布优化水和氢的吸附自由能,加深了对 Ru@Ni-MOF 优异 HER 性能的机理理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号