Journal of the American College of Cardiology ( IF 21.7 ) Pub Date : 2021-08-23 , DOI: 10.1016/j.jacc.2021.06.040 Marat Fudim 1 , William T Abraham 2 , Ralph Stephan von Bardeleben 3 , JoAnn Lindenfeld 4 , Piotr P Ponikowski 5 , Husam M Salah 6 , Muhammad Shahzeb Khan 7 , Horst Sievert 8 , Gregg W Stone 9 , Stefan D Anker 10 , Javed Butler 7
|
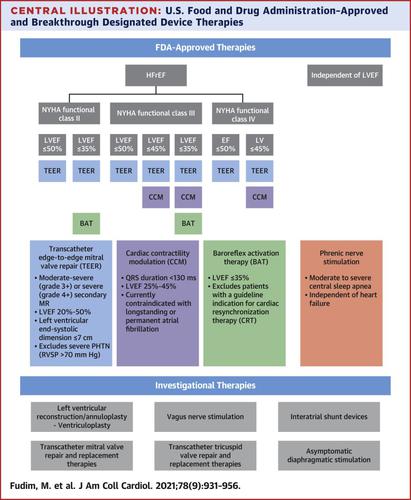
The regulatory landscape for device-based heart failure (HF) therapies has seen a major shift in the last 7 years. In 2013, the U.S. Food and Drug Administration released guidance for early feasibility and first-in-human studies, thereby encouraging device innovation, and in 2016 the U.S. Congress authorized the Breakthrough Devices Program to expedite access for Americans to innovative devices indicated for diagnosis and treatment of serious illnesses, such as HF. Since December 2016, there has been an increase in the number of HF devices for which manufacturers are seeking approval through the breakthrough designation pathway. This has led to a rapid uptake in the development and evaluation of device-based HF therapies. This article reviews the current and future landscape of device therapies for chronic HF and associated comorbidities and the regulatory environment that is driving current and future innovation.
中文翻译:

慢性心力衰竭的装置治疗
基于器械的心力衰竭 (HF) 疗法的监管环境在过去 7 年中发生了重大转变。2013 年,美国食品和药物管理局发布了早期可行性和首次人体研究指南,从而鼓励设备创新,2016 年美国国会授权突破性设备计划,以加快美国人获得用于诊断和治疗的创新设备。治疗严重疾病,例如 HF。自 2016 年 12 月以来,制造商通过突破性指定途径寻求批准的 HF 设备数量有所增加。这导致了基于设备的 HF 疗法的开发和评估的快速发展。









































 京公网安备 11010802027423号
京公网安备 11010802027423号