Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2021-08-24 , DOI: 10.1016/j.jorganchem.2021.122047 Tanmoy Mandal 1 , Sudha Yadav 2 , Joyanta Choudhury 1
|
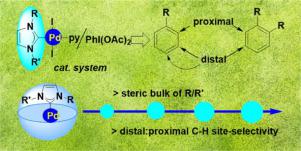
Although there has been a lot of progress in oxidative arene C–H functionalization reactions catalyzed by Pd(II/IV) system, the non-directed, site-selective functionalization of arene molecules is still challenging. It has been established that ligands play a pivotal role in controlling rate- as well as selectivity-determining step in a catalytic cycle involving well-defined metal-ligand bonding. N-heterocyclic carbene (NHC) ligands have had a tremendous contribution in the recent extraordinary success of achieving high reactivity and excellent selectivity in many catalytic processes including cross-coupling and olefin-metathesis reactions. However, the immense potential of these NHC ligands in improving site-selectivity of non-directed catalytic C–H functionalization reactions of simple arenes is yet to be realized, where overriding the electronic bias on deciding selectivity is a burdensome task. The presented work demonstrated an initiative step in this regard. Herein, a series of well-defined discrete [Pd()(py)I2] complexes with systematically varied degree of spatial congestion at the Pd centre, exerted through the R and R’ substituents on the NHC ligand, were explored in controlling the activity as well as the site-selectivity of non-directed acetoxylation of representative monosubstituted and disubstituted simple arenes (such as toluene, iodobenzene and bromobenzene, naphthalene and 1,2-dichlorobenzene). The resulting best yields were found to be 75% for toluene and 65% for bromobenzene with [Pd()(py)I2], 75% for iodobenzene and 79% for naphthalene with [Pd()(py)I2], and 41% for 1,2-dichlorobenzene with [Pd()(py)I2]. Most importantly, with increasing the bulkiness of the NHC ligand in the complexes, the selectivity of the distal C-acetoxylated products in comparison to the proximal ones, was enhanced to a great extent in all cases. Considering the vast library of NHC ligands, this study underscores the future opportunity to develop more strategies to improve the activity and the crucial site-selectivity of C–H functionalization reactions in simple as well as complex organic molecules.
中文翻译:

NHC配体在Pd(II)-NHC催化的简单芳烃非定向C-H乙酰氧基化中的空间效应
尽管在 Pd(II/IV) 体系催化的氧化芳烃 C-H 官能化反应方面取得了很多进展,但芳烃分子的非定向、位点选择性官能化仍然具有挑战性。已经确定配体在控制涉及明确金属配体键合的催化循环中的速率和选择性决定步骤中起着关键作用。N-杂环卡宾 (NHC) 配体在最近在许多催化过程(包括交叉偶联和烯烃复分解反应)中实现高反应性和优异选择性的非凡成功中做出了巨大贡献。然而,这些 NHC 配体在提高简单芳烃的非定向催化 C-H 官能化反应的位点选择性方面的巨大潜力尚未实现,在决定选择性时克服电子偏见是一项繁重的任务。所介绍的工作表明在这方面采取了主动措施。这里,一系列定义明确的离散 [Pd()(py)I 2 ] 配合物在 Pd 中心具有不同程度的空间拥塞,通过 NHC 配体上的 R 和 R' 取代基施加,在控制活性以及非定向的位点选择性方面进行了探索代表性的单取代和双取代的简单芳烃(如甲苯、碘苯和溴苯、萘和 1,2-二氯苯)的乙酰氧基化。发现所得的最佳产率是甲苯的 75% 和溴苯的 65% 与 [Pd()(py)I 2 ],75% 的碘苯和 79% 的萘和 [Pd()(py)I 2 ],1,2-二氯苯与 [Pd()(py)I 2 ]。最重要的是,随着复合物中 NHC 配体体积的增加,与近端 C-乙酰氧基化产物相比,远端 C-乙酰氧基化产物的选择性在所有情况下都有很大程度的提高。考虑到庞大的 NHC 配体库,这项研究强调了未来开发更多策略以提高简单和复杂有机分子中 C-H 官能化反应的活性和关键位点选择性的机会。









































 京公网安备 11010802027423号
京公网安备 11010802027423号