当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid–Liquid Phase Equilibria of the Quaternary system Li2SO4–LiBO2–Li2B4O7–H2O and the Ternary Subsystem LiBO2–Li2B4O7–H2O at T = 288.15 K and p = 0.1 MPa
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-24 , DOI: 10.1021/acs.jced.1c00258 Xiuxiu Yang 1 , Lele Chen 1 , Yafei Guo 1 , Dan Li 1, 2 , Tianlong Deng 1 , Lingzong Meng 1, 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-24 , DOI: 10.1021/acs.jced.1c00258 Xiuxiu Yang 1 , Lele Chen 1 , Yafei Guo 1 , Dan Li 1, 2 , Tianlong Deng 1 , Lingzong Meng 1, 2
Affiliation
|
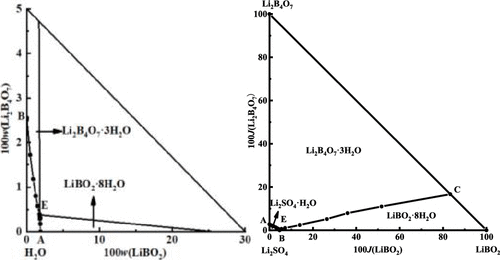
The solubilities, densities, refractive indices, and pH values in the systems LiBO2–Li2B4O7–H2O and Li2SO4–LiBO2–Li2B4O7–H2O at 288.15 K were studied by the classical dissolution method in this paper. The solid–liquid equilibrium phase diagrams and the physicochemical property diagrams were constructed with the experimental results. The phase diagram of the ternary system is composed of two single-salt crystallizations of LiBO2·8H2O and Li2B4O7·3H2O, two solubility univariant curves, and one invariant point saturated with the above salts. The comparison for the phase diagrams in the ternary system at different temperatures shows that the solubilities of lithium metaborate and lithium tetraborate in this system are in a positive correlation with the temperature. The dry-salt diagram of the quaternary system consists of three single-salt crystallization fields for Li2SO4·H2O, LiBO2·8H2O, and Li2B4O7·3H2O, three univariant solubility curves, and one invariant point. The physicochemical properties in the two systems change regularly as the concentration increases in solution. The calculated densities and refractive indices with empirical equations are in good agreement with the experimental results.
中文翻译:

四元系统 Li2SO4–LiBO2–Li2B4O7–H2O 和三元子系统 LiBO2–Li2B4O7–H2O 在 T = 288.15 K 和 p = 0.1 MPa 时的固液相平衡
LiBO 2 –Li 2 B 4 O 7 –H 2 O 和 Li 2 SO 4 –LiBO 2 –Li 2 B 4 O 7 –H 2 O 体系在 288.15 K 下的溶解度、密度、折射率和 pH 值为本文采用经典溶解法进行研究。结合实验结果构建了固液平衡相图和理化性质图。三元体系的相图由LiBO 2 ·8H 2 O和Li 2 B的两种单盐结晶组成4 O 7 ·3H 2 O,两条溶解度单变曲线,一个不变点被上述盐饱和。不同温度下三元体系相图的比较表明,偏硼酸锂和四硼酸锂在该体系中的溶解度与温度呈正相关。四元系干盐图由Li 2 SO 4 ·H 2 O、LiBO 2 ·8H 2 O和Li 2 B 4 O 7 ·3H 2三个单盐结晶场组成O,三个单变量溶解度曲线和一个不变点。随着溶液中浓度的增加,这两个系统的理化性质有规律地变化。用经验方程计算的密度和折射率与实验结果非常吻合。
更新日期:2021-09-09
中文翻译:

四元系统 Li2SO4–LiBO2–Li2B4O7–H2O 和三元子系统 LiBO2–Li2B4O7–H2O 在 T = 288.15 K 和 p = 0.1 MPa 时的固液相平衡
LiBO 2 –Li 2 B 4 O 7 –H 2 O 和 Li 2 SO 4 –LiBO 2 –Li 2 B 4 O 7 –H 2 O 体系在 288.15 K 下的溶解度、密度、折射率和 pH 值为本文采用经典溶解法进行研究。结合实验结果构建了固液平衡相图和理化性质图。三元体系的相图由LiBO 2 ·8H 2 O和Li 2 B的两种单盐结晶组成4 O 7 ·3H 2 O,两条溶解度单变曲线,一个不变点被上述盐饱和。不同温度下三元体系相图的比较表明,偏硼酸锂和四硼酸锂在该体系中的溶解度与温度呈正相关。四元系干盐图由Li 2 SO 4 ·H 2 O、LiBO 2 ·8H 2 O和Li 2 B 4 O 7 ·3H 2三个单盐结晶场组成O,三个单变量溶解度曲线和一个不变点。随着溶液中浓度的增加,这两个系统的理化性质有规律地变化。用经验方程计算的密度和折射率与实验结果非常吻合。









































 京公网安备 11010802027423号
京公网安备 11010802027423号