Chem Catalysis ( IF 11.5 ) Pub Date : 2021-08-24 , DOI: 10.1016/j.checat.2021.08.001 Di Si 1 , Bingyan Xiong 1 , Lisong Chen 1 , Jianlin Shi 2
|
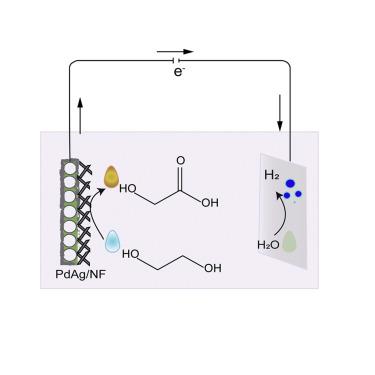
Armed with both hydroxyl and carboxyl groups, glycolic acid is an especially high-value-added chemical applied in diverse areas. However, its large-scale production suffers heavily from harsh synthetic conditions and poor selectivity. Here, an electrocatalytic approach has been developed for glycolic-acid production by electro-oxidation of ethylene glycol in alkaline solution on a PdAg electrocatalyst in situ grown on nickel foam (PdAg/NF). Resultantly, as low as 0.57 V versus reversible hydrogen electrode at 10 mA cm−2 is needed for ethylene glycol electro-oxidation, which is markedly (980 mV) lower than that of oxygen evolution in the same electrolyte without ethylene glycol. Moreover, glycolic acid can be electro-catalytically produced most efficiently at an extremely high current density of 300 mA cm−2 without the occurrence of oxygen evolution. When coupled with a Pt cathodic catalyst, a cell potential of 1.02 V is necessary for cathodic hydrogen production at faradic efficiency of 100%.
中文翻译:

乙醇酸与析氢耦合的高选择性和高效电催化合成
乙醇酸同时具有羟基和羧基,是一种应用在不同领域的高附加值化学品。然而,其大规模生产受到合成条件苛刻和选择性差的严重影响。在这里,通过在镍泡沫 (PdAg/NF) 上原位生长的 PdAg 电催化剂上,通过乙二醇在碱性溶液中的电氧化,开发了一种用于乙醇酸生产的电催化方法。结果,相对于 10 mA cm -2 的可逆氢电极低至 0.57 V乙二醇电氧化需要 ,其显着(980 mV)低于不含乙二醇的相同电解质中的氧气析出量。此外,乙醇酸可以在300 mA cm -2的极高电流密度下最有效地电催化生产,而不会发生氧析出。当与 Pt 阴极催化剂结合使用时,1.02 V 的电池电位对于以 100% 的法拉第效率进行阴极制氢是必要的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号