Molecular Cell ( IF 14.5 ) Pub Date : 2021-08-24 , DOI: 10.1016/j.molcel.2021.07.033 Xiaojing Cong 1 , Damien Maurel 1 , Hélène Déméné 2 , Ieva Vasiliauskaité-Brooks 1 , Joanna Hagelberger 1 , Fanny Peysson 1 , Julie Saint-Paul 1 , Jérôme Golebiowski 3 , Sébastien Granier 1 , Rémy Sounier 1
|
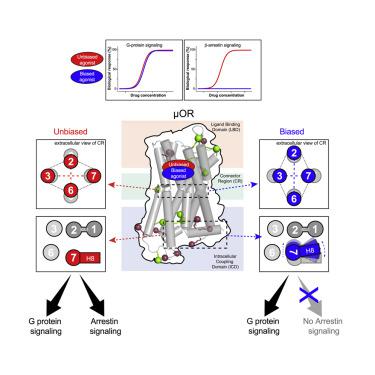
GPCR functional selectivity opens new opportunities for the design of safer drugs. Ligands orchestrate GPCR signaling cascades by modulating the receptor conformational landscape. Our study provides insights into the dynamic mechanism enabling opioid ligands to preferentially activate the G protein over the β-arrestin pathways through the μ-opioid receptor (μOR). We combine functional assays in living cells, solution NMR spectroscopy, and enhanced-sampling molecular dynamic simulations to identify the specific μOR conformations induced by G protein-biased agonists. In particular, we describe the dynamic and allosteric communications between the ligand-binding pocket and the receptor intracellular domains, through conserved motifs in class A GPCRs. Most strikingly, the biased agonists trigger μOR conformational changes in the intracellular loop 1 and helix 8 domains, which may impair β-arrestin binding or signaling. The findings may apply to other GPCR families and provide key molecular information that could facilitate the design of biased ligands.
中文翻译:

μ-阿片受体偏向信号机制的分子见解
GPCR 功能选择性为设计更安全的药物开辟了新机遇。配体通过调节受体构象景观来协调 GPCR 信号级联。我们的研究提供了对使阿片类药物配体通过 μ-阿片受体 (μOR) 优先激活 G 蛋白而不是 β-抑制蛋白途径的动态机制的见解。我们将活细胞中的功能分析、溶液核磁共振光谱和增强采样分子动力学模拟结合起来,以确定由 G 蛋白偏向激动剂诱导的特定 μOR 构象。特别是,我们通过 A 类 GPCR 中的保守基序描述了配体结合口袋和受体胞内结构域之间的动态和变构通信。最引人注目的是,偏向激动剂触发细胞内环 1 和螺旋 8 结构域中的 μOR 构象变化,这可能会损害 β-arrestin 结合或信号传导。这些发现可能适用于其他 GPCR 家族,并提供有助于设计偏向配体的关键分子信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号