Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Complexation in situ of 1-methylpiperidine, 1,2-dimethylpyrrolidin, and 1,2-dimethylpiperidine with rhodium(II) tetracarboxylates: Nuclear magnetic resonance spectroscopy, chiral recognition, and density functional theory studies
Chirality ( IF 2.8 ) Pub Date : 2021-08-23 , DOI: 10.1002/chir.23345 Agnieszka Sadlej 1 , Jarosław Jaźwiński 1
Chirality ( IF 2.8 ) Pub Date : 2021-08-23 , DOI: 10.1002/chir.23345 Agnieszka Sadlej 1 , Jarosław Jaźwiński 1
Affiliation
|
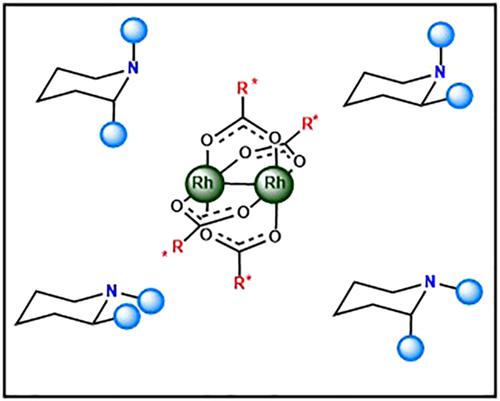
Complexation in situ of 1-methylpiperidine, racemic 1,2-dimethylpyrrolidin, and racemic 1,2-dimethylpiperidine with rhodium(II) tetracarboxylates in chloroform was studied by 1H and 13C nuclear magnetic resonance (NMR) spectroscopy and density functional theory (DFT) methods. As substrates, three dirhodium(II) compounds were applied, tetraacetate, tetrakistrifluoroacetate, and a derivative of optically pure Mosher's acid. Due to conformational flexibility, free and complexed ligands can adopt potentially various conformations. The NMR titration experiments revealed the subsequent formation of 1:1 and 1:2 complexes, depending on the molar ratio of substrate to ligand. Conformations of free and complexed ligands were examined by the comparison of experimental and DFT gauge-independent atomic orbital (GIAO) calculated chemical shifts and by the analysis of the internal energy of the compounds. For some ligand and substrate combinations, a mixture of complexes differing in ligand conformations was formed. Complexes of Mosher's acid derivative of rhodium(II) with racemic 1,2-dimethylpyrrolidin and 1,2-dimethylpiperidine exhibited NMR chiral recognition phenomenon, manifested by splitting of signals in 13C NMR and 1H,13C HSQC spectra.
中文翻译:

1-甲基哌啶、1,2-二甲基吡咯烷和 1,2-二甲基哌啶与四羧酸铑 (II) 的原位络合:核磁共振光谱、手性识别和密度泛函理论研究
通过1 H 和13研究了1-甲基哌啶、外消旋 1,2-二甲基吡咯烷和外消旋 1,2-二甲基哌啶与四羧酸铑 (II) 在氯仿中的原位络合C 核磁共振 (NMR) 光谱和密度泛函理论 (DFT) 方法。作为基材,应用了三种二铑 (II) 化合物:四乙酸盐、四三氟乙酸盐和光学纯的 Mosher 酸衍生物。由于构象的灵活性,游离的和复合的配体可以采用潜在的各种构象。NMR 滴定实验揭示了 1:1 和 1:2 配合物的后续形成,这取决于底物与配体的摩尔比。通过比较实验和 DFT 规范独立原子轨道 (GIAO) 计算的化学位移和分析化合物的内能,检查了游离和络合配体的构象。对于一些配体和底物组合,形成了配体构象不同的复合物混合物。13 C NMR 和1 H、13 C HSQC 光谱。
更新日期:2021-09-17
中文翻译:

1-甲基哌啶、1,2-二甲基吡咯烷和 1,2-二甲基哌啶与四羧酸铑 (II) 的原位络合:核磁共振光谱、手性识别和密度泛函理论研究
通过1 H 和13研究了1-甲基哌啶、外消旋 1,2-二甲基吡咯烷和外消旋 1,2-二甲基哌啶与四羧酸铑 (II) 在氯仿中的原位络合C 核磁共振 (NMR) 光谱和密度泛函理论 (DFT) 方法。作为基材,应用了三种二铑 (II) 化合物:四乙酸盐、四三氟乙酸盐和光学纯的 Mosher 酸衍生物。由于构象的灵活性,游离的和复合的配体可以采用潜在的各种构象。NMR 滴定实验揭示了 1:1 和 1:2 配合物的后续形成,这取决于底物与配体的摩尔比。通过比较实验和 DFT 规范独立原子轨道 (GIAO) 计算的化学位移和分析化合物的内能,检查了游离和络合配体的构象。对于一些配体和底物组合,形成了配体构象不同的复合物混合物。13 C NMR 和1 H、13 C HSQC 光谱。









































 京公网安备 11010802027423号
京公网安备 11010802027423号