Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2021-08-23 , DOI: 10.1016/j.jorganchem.2021.122042 Qinghua Ren 1 , Dongtao Zhang 1 , Lin Zheng 1
|
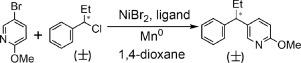
The detail mechanisms of enantioselective Ni-catalyzed reductive coupling of 5-bromide-2-methoxypyridine (R1) and (1-chloropropyl) benzene (R2) to form 1,1-diarylalkanes (R, S)-P were studied using Density Functional Theory calculations. The overall catalytic cycles include the following basic steps: the oxidative addition, reduction, radical production, radical addition, reductive elimination and the catalyst regeneration. Our calculated results show that the favored way from the reactant (S)-R2 to get the products (R, S)-P is Path C (the NiI catalyst initially combined with alkyl chloride) where the energy barrier of the rate-determining step is the oxidative addition step with 9.1 kcal/mol (of TS1b), but the favored path starting from the reactant (R)-R2 to get the products (R, S)-P is Path A (the NiI catalyst initially combined with aryl bromide) or Path B (the Ni° catalyst initially combined with the aryl bromide) where the energy barrier of the rate-determining step is the radical production step with 12.7 kcal/mol (of (R)-TS2). Our calculated results also indicate that the formation of (R)-P is dominant over the product (S)-P, which meets the experimental results. The ligand effects have also been studied.
中文翻译:

对映选择性镍催化还原偶联反应生成 1,1-二芳基烷烃机理的 DFT 研究
使用对映选择性镍催化还原偶联 5-溴-2-甲氧基吡啶 ( R 1 ) 和 (1-氯丙基) 苯 ( R 2 )形成 1,1-二芳基烷烃( R, S )-P的详细机理研究密度泛函理论计算。整个催化循环包括以下基本步骤:氧化加成、还原、自由基产生、自由基加成、还原消除和催化剂再生。我们的计算结果表明,从反应物( S )-R 2获得产物( R, S )-P的有利途径是路径 C(Ni I催化剂最初与烷基氯结合),其中限速步骤的能量势垒是氧化加成步骤,具有 9.1 kcal/mol(TS 1b),但从反应物( R )-R 2开始的有利路径得到产物( R, S )-P是路径 A(Ni I催化剂最初与芳基溴化物结合)或路径 B(Ni°催化剂最初与芳基溴化物结合),其中速率决定步骤的能垒是自由基12.7 kcal/mol ( ( R )-TS 2 )的生产步骤。我们的计算结果还表明( R)-P比乘积( S )-P占优势,符合实验结果。还研究了配体效应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号