当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A cobalt oxide–polypyrrole nanocomposite as an efficient and stable electrode material for electrocatalytic water oxidation
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2021-08-05 , DOI: 10.1039/d1se00363a Daniela V. Morales 1, 2 , Catalina N. Astudillo 1 , Veronica Anastasoaie 1, 3 , Baptiste Dautreppe 1 , Bruno F. Urbano 4 , Bernabé L. Rivas 4 , Chantal Gondran 1 , Dmitry Aldakov 5 , Benoit Chovelon 6, 7 , Dominique André 6 , Jean-Luc Putaux 8 , Christine Lancelon-Pin 8 , Selim Sirach 1 , Eleonora-Mihaela Ungureanu 3 , Cyrille Costentin 1, 9 , Marie-Noëlle Collomb 1 , Jérôme Fortage 1
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2021-08-05 , DOI: 10.1039/d1se00363a Daniela V. Morales 1, 2 , Catalina N. Astudillo 1 , Veronica Anastasoaie 1, 3 , Baptiste Dautreppe 1 , Bruno F. Urbano 4 , Bernabé L. Rivas 4 , Chantal Gondran 1 , Dmitry Aldakov 5 , Benoit Chovelon 6, 7 , Dominique André 6 , Jean-Luc Putaux 8 , Christine Lancelon-Pin 8 , Selim Sirach 1 , Eleonora-Mihaela Ungureanu 3 , Cyrille Costentin 1, 9 , Marie-Noëlle Collomb 1 , Jérôme Fortage 1
Affiliation
|
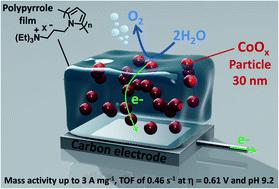
Developing electrolyzers operating under neutral or near-neutral conditions with catalysts based only on earth-abundant metals is highly desirable with a view to reduce the cost of hydrogen production from the water splitting reaction and avoid the environmental issues related to corrosion usually encountered with alkaline electrolyzers. Herein, we report a highly active and stable anode material for the oxygen evolution reaction (OER) under mild-pH conditions based on cobalt oxide-nanoparticles embedded into a poly(pyrrole-alkylammonium) matrix (denoted as PPN+-CoOx). Examples of hybrid materials combining metal oxide nanoparticles as OER catalysts within a polymer film are still rare. However, they are very promising to control the formation and the size of metal particles in view of enhancing the electrochemically active surface area and thus the electrocatalytic performances. Our strategy consists in electroprecipitating Co0 nanoparticles by the reduction of an anionic cobalt oxalate complex into a cationic PPN+ film, the latter being previously deposited onto an electrode surface by electropolymerization. The Co0 nanoparticles within the composite are then partially in situ oxidized under air exposure to CoO, and then finally fully oxidized to CoOx by successive scans between 0 and 1.2 V vs. Ag/AgCl in a borate buffer at pH 9.2. This nanocomposite material is highly structured with around 30 nm-large CoOx nanoparticles well dispersed into the polypyrrole film conferring a high OER electrocatalytic activity at a near neutral pH of 9.2 with exceptional values of mass activity and turnover frequency of 3.01 A mg−1 and 0.46 s−1 respectively, at an overpotential of 0.61 V and with a cobalt loading of 1.34 μg cm−2. These performances place the PPN+-CoOx electrode among the most active anodes described in the literature employing cobalt oxide under mild pH conditions. In addition, when the PPN+-CoOx material is electrodeposited on carbon paper with a higher roughness than a simple carbon electrode, the physisorption of the film on the electrode is considerably enhanced resulting in a stable catalytic current for over more than 43 h. Post electrolysis characterization by SEM and EDX confirms the integrity of the PPN+-CoOx material after many hours of electrocatalysis. This demonstrates the beneficial role of the polypyrrole matrix in the achievement of very stable and highly active anodes for water oxidation.
中文翻译:

氧化钴-聚吡咯纳米复合材料作为一种高效稳定的电催化水氧化电极材料
开发在中性或近中性条件下使用仅基于地球丰富金属的催化剂的电解槽是非常可取的,以降低水分解反应制氢的成本并避免碱性电解槽通常遇到的与腐蚀相关的环境问题. 在此,我们报告了一种基于嵌入聚(吡咯-烷基铵)基质中的氧化钴纳米颗粒(表示为 PPN + -CoO x)。在聚合物薄膜中结合金属氧化物纳米颗粒作为 OER 催化剂的混合材料的例子仍然很少见。然而,鉴于提高电化学活性表面积和电催化性能,它们非常有希望控制金属颗粒的形成和尺寸。我们的策略包括通过将阴离子草酸钴络合物还原成阳离子 PPN +膜来电沉淀Co 0纳米粒子,后者预先通过电聚合沉积到电极表面上。复合材料中的 Co 0纳米颗粒然后在暴露于 CoO 的空气中部分原位氧化,最后完全氧化为 CoO x通过在 pH 9.2 的硼酸盐缓冲液中在0 到 1.2 V与Ag/AgCl之间连续扫描。这种纳米复合材料高度结构化,其中约 30 nm 大的 CoO x纳米颗粒很好地分散在聚吡咯膜中,在接近中性 pH 值 9.2 时具有高 OER 电催化活性,质量活性值和周转频率为 3.01 A mg -1和分别为0.46 s -1,过电位为0.61 V,钴负载量为1.34 μg cm -2。这些性能使 PPN + -CoO x电极成为文献中描述的在温和 pH 条件下使用氧化钴的最活跃的阳极之一。此外,当 PPN +-CoO x材料电沉积在碳纸上,其粗糙度比简单的碳电极更高,电极上薄膜的物理吸附显着增强,导致稳定的催化电流超过 43 小时。SEM 和 EDX 的电解后表征证实了 PPN + -CoO x材料在电催化数小时后的完整性。这证明了聚吡咯基质在实现非常稳定和高活性的水氧化阳极方面的有益作用。
更新日期:2021-08-23
中文翻译:

氧化钴-聚吡咯纳米复合材料作为一种高效稳定的电催化水氧化电极材料
开发在中性或近中性条件下使用仅基于地球丰富金属的催化剂的电解槽是非常可取的,以降低水分解反应制氢的成本并避免碱性电解槽通常遇到的与腐蚀相关的环境问题. 在此,我们报告了一种基于嵌入聚(吡咯-烷基铵)基质中的氧化钴纳米颗粒(表示为 PPN + -CoO x)。在聚合物薄膜中结合金属氧化物纳米颗粒作为 OER 催化剂的混合材料的例子仍然很少见。然而,鉴于提高电化学活性表面积和电催化性能,它们非常有希望控制金属颗粒的形成和尺寸。我们的策略包括通过将阴离子草酸钴络合物还原成阳离子 PPN +膜来电沉淀Co 0纳米粒子,后者预先通过电聚合沉积到电极表面上。复合材料中的 Co 0纳米颗粒然后在暴露于 CoO 的空气中部分原位氧化,最后完全氧化为 CoO x通过在 pH 9.2 的硼酸盐缓冲液中在0 到 1.2 V与Ag/AgCl之间连续扫描。这种纳米复合材料高度结构化,其中约 30 nm 大的 CoO x纳米颗粒很好地分散在聚吡咯膜中,在接近中性 pH 值 9.2 时具有高 OER 电催化活性,质量活性值和周转频率为 3.01 A mg -1和分别为0.46 s -1,过电位为0.61 V,钴负载量为1.34 μg cm -2。这些性能使 PPN + -CoO x电极成为文献中描述的在温和 pH 条件下使用氧化钴的最活跃的阳极之一。此外,当 PPN +-CoO x材料电沉积在碳纸上,其粗糙度比简单的碳电极更高,电极上薄膜的物理吸附显着增强,导致稳定的催化电流超过 43 小时。SEM 和 EDX 的电解后表征证实了 PPN + -CoO x材料在电催化数小时后的完整性。这证明了聚吡咯基质在实现非常稳定和高活性的水氧化阳极方面的有益作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号