当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Escherichia coli K-12 has two distinguishable PriA-PriB replication restart pathways
Molecular Microbiology ( IF 2.6 ) Pub Date : 2021-08-23 , DOI: 10.1111/mmi.14802 Steven J Sandler 1 , Maxime Leroux 1, 2 , Tricia A Windgassen 3, 4 , James L Keck 3
Molecular Microbiology ( IF 2.6 ) Pub Date : 2021-08-23 , DOI: 10.1111/mmi.14802 Steven J Sandler 1 , Maxime Leroux 1, 2 , Tricia A Windgassen 3, 4 , James L Keck 3
Affiliation
|
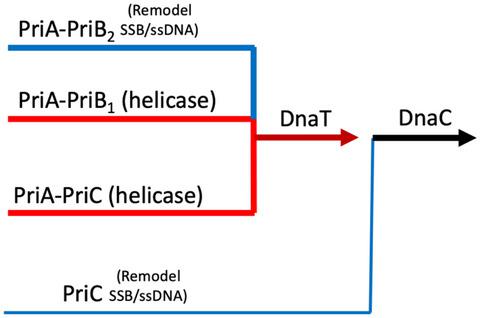
In Escherichia coli, PriA, PriB, PriC, and DnaT proteins mediate three pathways for Replication Restart called PriA-PriB, PriA-PriC, and PriC. PriA is crucial for two of the three pathways. Its absence leads to slow growth, high basal levels of SOS expression, poorly partitioning nucleoids, UV sensitivity, and recombination deficiency. PriA has ATPase and helicase activities and interacts with PriB, DnaT, and single-stranded DNA-binding protein (SSB). priA300 (K230R) and priA301 (C479Y) have no phenotype as single mutants, but each phenocopy a priA-null mutant combined with ∆priB. This suggested that the two priA mutations affected the helicase activity that is required for the PriA-PriC pathway. To further test this, the biochemical activities of purified PriA300 and PriA301 were examined. As expected, PriA300 lacks ATPase and helicase activities but retains the ability to interact with PriB. PriA301, however, retains significant PriB-stimulated helicase activity even though PriA301 interactions with PriB and DNA are weakened. A PriA300,301 variant retains only the ability to interact with DNA in vitro and phenocopies the priA-null phenotype in vivo. This suggests that there are two biochemically and genetically distinct PriA-PriB pathways. One uses PriB-stimulated helicase activity to free a region of ssDNA and the other uses helicase-independent remodeling activity.
中文翻译:

大肠杆菌 K-12 具有两种可区分的 PriA-PriB 复制重启途径
在大肠杆菌中,PriA、PriB、PriC 和 DnaT 蛋白介导复制重启的三种途径,称为 PriA-PriB、PriA-PriC 和 PriC。PriA 对于三个途径中的两个至关重要。它的缺失导致生长缓慢、SOS 表达的基础水平高、类核分配不良、紫外线敏感性和重组缺陷。PriA 具有 ATPase 和解旋酶活性,并与 PriB、DnaT 和单链 DNA 结合蛋白 (SSB) 相互作用。priA300 (K230R) 和priA301 (C479Y) 没有作为单一突变体的表型,但每个 phenocopy 一个priA -null 突变体与 Δ priB结合。这表明两个priA突变影响了 PriA-PriC 途径所需的解旋酶活性。为了进一步测试这一点,检测了纯化的 PriA300 和 PriA301 的生化活性。正如预期的那样,PriA300 缺乏 ATPase 和解旋酶活性,但保留了与 PriB 相互作用的能力。然而,即使 PriA301 与 PriB 和 DNA 的相互作用减弱,PriA301 仍保留显着的 PriB 刺激的解旋酶活性。PriA300,301 变体仅保留在体外与 DNA 相互作用的能力,并在体内对priA- null 表型进行表型复制。这表明存在两种生化和遗传上不同的 PriA-PriB 途径。一种使用 PriB 刺激的解旋酶活性来释放 ssDNA 区域,另一种使用不依赖解旋酶的重塑活性。
更新日期:2021-10-25
中文翻译:

大肠杆菌 K-12 具有两种可区分的 PriA-PriB 复制重启途径
在大肠杆菌中,PriA、PriB、PriC 和 DnaT 蛋白介导复制重启的三种途径,称为 PriA-PriB、PriA-PriC 和 PriC。PriA 对于三个途径中的两个至关重要。它的缺失导致生长缓慢、SOS 表达的基础水平高、类核分配不良、紫外线敏感性和重组缺陷。PriA 具有 ATPase 和解旋酶活性,并与 PriB、DnaT 和单链 DNA 结合蛋白 (SSB) 相互作用。priA300 (K230R) 和priA301 (C479Y) 没有作为单一突变体的表型,但每个 phenocopy 一个priA -null 突变体与 Δ priB结合。这表明两个priA突变影响了 PriA-PriC 途径所需的解旋酶活性。为了进一步测试这一点,检测了纯化的 PriA300 和 PriA301 的生化活性。正如预期的那样,PriA300 缺乏 ATPase 和解旋酶活性,但保留了与 PriB 相互作用的能力。然而,即使 PriA301 与 PriB 和 DNA 的相互作用减弱,PriA301 仍保留显着的 PriB 刺激的解旋酶活性。PriA300,301 变体仅保留在体外与 DNA 相互作用的能力,并在体内对priA- null 表型进行表型复制。这表明存在两种生化和遗传上不同的 PriA-PriB 途径。一种使用 PriB 刺激的解旋酶活性来释放 ssDNA 区域,另一种使用不依赖解旋酶的重塑活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号