Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2021-08-23 , DOI: 10.1016/j.bmc.2021.116376 Yichang Ren 1 , Yong Ruan 1 , Binbin Cheng 2 , Ling Li 1 , Jin Liu 1 , Yuyu Fang 3 , Jianjun Chen 1
|
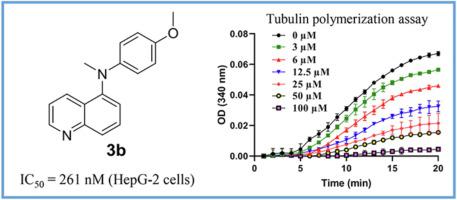
A series of acridine and quinoline derivatives were designed and synthesized based on our previous work as novel tubulin inhibitors targeting the colchicine binding site. Among them, compound 3b exhibited the highest antiproliferative activity with an IC50 of 261 nM against HepG-2 cells (the most sensitive cell line). In addition, compound 3b was able to suppress the formation of HepG-2 colonies. Mechanism studies revealed that compound 3b effectively inhibited tubulin polymerization in vitro and disrupted microtubule dynamics in HepG-2 cells. Furthermore, compound 3b inhibited the migration of cancer cells in a dose dependent manner. Moreover, compound 3b induced cell cycle arrest in G2/M phase and led to cell apoptosis. Finally, docking studies demonstrated that compound 3b fitted nicely in the colchicine binding site of tubulin and overlapped well with CA-4. Collectively, these results suggested that compound 3b represents a novel tubulin inhibitor deserving further investigation.
中文翻译:

作为具有抗癌活性的微管蛋白聚合抑制剂的新型吖啶和喹啉衍生物的设计、合成和生物学评价
一系列吖啶和喹啉衍生物是基于我们之前的工作设计和合成的,它们是针对秋水仙碱结合位点的新型微管蛋白抑制剂。其中,化合物3b对HepG-2细胞(最敏感的细胞系)表现出最高的抗增殖活性,IC 50为261 nM。此外,化合物3b能够抑制 HepG-2 集落的形成。机制研究表明,化合物3b在体外有效抑制微管蛋白聚合并破坏 HepG-2 细胞中的微管动力学。此外,化合物3b以剂量依赖性方式抑制癌细胞的迁移。此外,化合物3b诱导细胞周期停滞在 G2/M 期并导致细胞凋亡。最后,对接研究表明,化合物3b与微管蛋白的秋水仙碱结合位点非常吻合,并与 CA-4 重叠良好。总的来说,这些结果表明化合物3b是一种值得进一步研究的新型微管蛋白抑制剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号