当前位置:
X-MOL 学术
›
Acta Cryst. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron(II)–alkoxide and –aryloxide complexes of a tris(thioether)borate ligand: synthesis, molecular structures, and implications on the origin of instability of their iron(II)–catecholate counterpart
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2021-08-23 , DOI: 10.1107/s2053229621008500 Peng Wang 1 , Glenn P A Yap 1 , Charles G Riordan 1
Acta Crystallographica Section C ( IF 0.7 ) Pub Date : 2021-08-23 , DOI: 10.1107/s2053229621008500 Peng Wang 1 , Glenn P A Yap 1 , Charles G Riordan 1
Affiliation
|
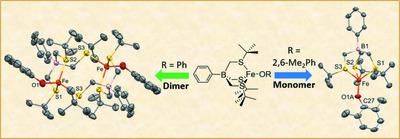
The phenyltris[(tert-butylthio)methyl]borate ligand, [PhTttBu], has been studied extensively as a platform for coordination, organometallic, and bioinorganic chemistry, especially with 3d metals. While [PhTttBu]Co(3,5-DBCatH) (3,5-DBCatH is 3,5-di-tert-butylcatecholate), a CoII–monoanionic catecholate complex, was successfully isolated to model the active site of cobalt(II)-substituted homoprotocatechuate 2,3-dioxygenase (Co-HPCD) [Wang et al. (2019). Inorg. Chim. Acta, 488, 49–55], its iron(II) counterpart, [PhTttBu]Fe(3,5-DBCatH), was not accessible via similar synthetic routes. Switching the nucleophile from catecholate to alkoxide or aryloxide, however, led to the successful isolation of three highly air-sensitive FeII–alkoxide and –aryloxide complexes, namely, (triphenylmethoxo){tris[(tert-butylsulfanyl)methyl]phenylborato-κ3S,S′,S′′}iron(II), [Fe(C21H38BS3)(C19H15O)], (2), (2,6-dimethylphenolato){tris[(tert-butylsulfanyl)methyl]phenylborato-κ3S,S′,S′′}iron(II), [Fe(C21H38BS3)(C8H9O)], (3), and bis{μ-tris[(tert-butylsulfanyl)methyl]phenylborato-κ3S,S′:S′′}bis[(phenolato-κO)iron(II)] toluene disolvate, [Fe2(C21H38BS3)2(C6H5O)2]·2C7H8, (4). In the solid state, compounds (2) and (3) are monomeric, with [PhTttBu] acting as a tridentate ligand. In contrast, compound (4) crystallizes as a dimeric complex, wherein each [PhTttBu] ligand binds to an iron centre with two thioethers and binds to the other iron centre with the third thioether. The molecular structures of (2)–(4) demonstrate a diversity in the binding modes of [PhTttBu] and highlight its potential use for assembling multinuclear complexes. In addition, the successful isolation of (2)–(4), as well as the structural information of a [PhTttBu] modification product, namely, bis{μ-tris[(tert-butylsulfanyl)methyl](2-oxidophenolato)borato-κO,O′,S,S′:O′}dicobalt(II), [Co2(C21H37BO2S3)2], (5), obtained from the reaction of [PhTttBu]CoCl with potassium monoanionic catecholate, shed light on the origin of the instability of [PhTttBu]Fe(3,5-DBCatH).
中文翻译:

三(硫醚)硼酸盐配体的铁(II)-醇盐和-芳基氧化物配合物:合成、分子结构及其对铁(II)-儿茶酚盐对应物不稳定性起源的影响
苯基三[(叔丁硫基)甲基]硼酸盐配体 [PhTt t Bu ] 作为配位、有机金属和生物无机化学的平台已被广泛研究,尤其是与 3 d金属。虽然 [PhTt t Bu ]Co(3,5-DBCatH)(3,5-DBCatH 是 3,5-二叔丁基儿茶酚酸酯),一种 Co II -单阴离子儿茶酚酸酯络合物,被成功分离以模拟钴的活性位点(II)-取代的高原儿茶酸 2,3-双加氧酶 (Co-HPCD) [Wang et al. (2019)。无机物。哼。Acta , 488 , 49–55],其铁 (II) 对应物,[PhTt t Bu]Fe(3,5-DBCatH),无法通过类似的合成路线获得。然而,将亲核试剂从儿茶酚盐转换为醇盐或芳氧化物,成功分离了三种高度对空气敏感的 Fe II -醇盐和 -芳氧化物复合物,即(三苯基甲氧基){三[(叔丁基硫烷基)甲基]苯基硼酸-κ 3 S , S ', S ''}铁(II), [Fe(C 21 H 38 BS 3 )(C 19 H 15 O)], (2), (2,6-二甲基苯酚){tris[( tert -丁基硫烷基)甲基]苯基硼酸根-κ 3 S , S ',S ''}铁(II)、[Fe(C 21 H 38 BS 3 )(C 8 H 9 O)]、(3)和双{μ-三[(叔丁基硫烷基)甲基]苯基硼酸-κ 3 S , S ': S ''}双[(苯酚-κ O )铁(II)]甲苯二溶剂化物,[Fe 2 (C 21 H 38 BS 3 ) 2 (C 6 H 5 O) 2 ]·2C 7 H 8、(4)。在固态下,化合物 (2) 和 (3) 是单体,具有 [PhTt t Bu] 作为三齿配体。相比之下,化合物 (4) 结晶为二聚复合物,其中每个 [PhTt t Bu ] 配体通过两个硫醚与一个铁中心结合,并通过第三个硫醚与另一个铁中心结合。(2)-(4) 的分子结构证明了 [PhTt t Bu ]结合模式的多样性,并突出了其组装多核复合物的潜在用途。此外,(2)-(4) 的成功分离,以及 [PhTt t Bu ] 改性产物的结构信息,即双{μ-三[(叔丁基硫烷基)甲基](2-氧化苯酚)硼酸-κ O , O ′, S , S': O '}dicobalt(II), [Co 2 (C 21 H 37 BO 2 S 3 ) 2 ], (5),由 [PhTt t Bu ]CoCl 与单阴离子儿茶酚钾反应获得,阐明了[PhTt t Bu ]Fe(3,5-DBCatH)不稳定性的起源。
更新日期:2021-09-06
中文翻译:

三(硫醚)硼酸盐配体的铁(II)-醇盐和-芳基氧化物配合物:合成、分子结构及其对铁(II)-儿茶酚盐对应物不稳定性起源的影响
苯基三[(叔丁硫基)甲基]硼酸盐配体 [PhTt t Bu ] 作为配位、有机金属和生物无机化学的平台已被广泛研究,尤其是与 3 d金属。虽然 [PhTt t Bu ]Co(3,5-DBCatH)(3,5-DBCatH 是 3,5-二叔丁基儿茶酚酸酯),一种 Co II -单阴离子儿茶酚酸酯络合物,被成功分离以模拟钴的活性位点(II)-取代的高原儿茶酸 2,3-双加氧酶 (Co-HPCD) [Wang et al. (2019)。无机物。哼。Acta , 488 , 49–55],其铁 (II) 对应物,[PhTt t Bu]Fe(3,5-DBCatH),无法通过类似的合成路线获得。然而,将亲核试剂从儿茶酚盐转换为醇盐或芳氧化物,成功分离了三种高度对空气敏感的 Fe II -醇盐和 -芳氧化物复合物,即(三苯基甲氧基){三[(叔丁基硫烷基)甲基]苯基硼酸-κ 3 S , S ', S ''}铁(II), [Fe(C 21 H 38 BS 3 )(C 19 H 15 O)], (2), (2,6-二甲基苯酚){tris[( tert -丁基硫烷基)甲基]苯基硼酸根-κ 3 S , S ',S ''}铁(II)、[Fe(C 21 H 38 BS 3 )(C 8 H 9 O)]、(3)和双{μ-三[(叔丁基硫烷基)甲基]苯基硼酸-κ 3 S , S ': S ''}双[(苯酚-κ O )铁(II)]甲苯二溶剂化物,[Fe 2 (C 21 H 38 BS 3 ) 2 (C 6 H 5 O) 2 ]·2C 7 H 8、(4)。在固态下,化合物 (2) 和 (3) 是单体,具有 [PhTt t Bu] 作为三齿配体。相比之下,化合物 (4) 结晶为二聚复合物,其中每个 [PhTt t Bu ] 配体通过两个硫醚与一个铁中心结合,并通过第三个硫醚与另一个铁中心结合。(2)-(4) 的分子结构证明了 [PhTt t Bu ]结合模式的多样性,并突出了其组装多核复合物的潜在用途。此外,(2)-(4) 的成功分离,以及 [PhTt t Bu ] 改性产物的结构信息,即双{μ-三[(叔丁基硫烷基)甲基](2-氧化苯酚)硼酸-κ O , O ′, S , S': O '}dicobalt(II), [Co 2 (C 21 H 37 BO 2 S 3 ) 2 ], (5),由 [PhTt t Bu ]CoCl 与单阴离子儿茶酚钾反应获得,阐明了[PhTt t Bu ]Fe(3,5-DBCatH)不稳定性的起源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号