当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Uncoupling Amphipathicity and Hydrophobicity: Role of Charge Clustering in Membrane Interactions of Cationic Antimicrobial Peptides
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-23 , DOI: 10.1021/acs.biochem.1c00367 Shelley He 1, 2 , Tracy A Stone 1, 2 , Charles M Deber 1, 2
Biochemistry ( IF 2.9 ) Pub Date : 2021-08-23 , DOI: 10.1021/acs.biochem.1c00367 Shelley He 1, 2 , Tracy A Stone 1, 2 , Charles M Deber 1, 2
Affiliation
|
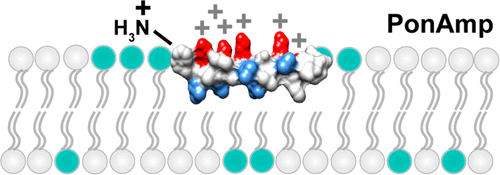
Peptides with a combination of high positive charge and high hydrophobicity have high antimicrobial activity, as epitomized by peptide venoms, which are designed by nature as disruptors of host membranes yet also display significant efficacy against pathogens. To investigate this phenomenon systematically, here we focus on ponericin W1, a peptide venom isolated from Pachycondyla goeldii ants (WLGSALKIGAKLLPSVVGLFKKKKQ) to examine whether Lys positioning can be broadly applied to optimize the functional range of existing natural sequences. We prepared sets of ponericin W1 analogues, where Lys residues were either distributed in an amphipathic manner throughout the sequence (PonAmp), clustered at the N-terminus (PonN), or clustered at the C-terminus (PonC), along with their counterparts of reduced hydrophobicity through 2–4 Leu-to-Ala replacements. We found that wild-type ponericin W1 and all three variants displayed toxicity against human erythrocytes, but hemolysis was eliminated by the replacement of two or more Leu residues by Ala residues. As well, peptides containing up to 3 Leu-to-Ala replacements retained antimicrobial activity against E. coli bacteria. Biophysical analyses of peptide–membrane interaction patterns by circular dichroism spectroscopy revealed a novel mode of cluster-dependent peptide positioning vis-à-vis the water–membrane interface, where PonAmp and PonC peptides displayed full or partial helical structures, while PonN peptides were unstructured, likely due, in part, to dynamic interchange between aqueous and membrane surface environments. The overall findings suggest that the lower membrane penetration of N-terminal charge-clustered constructs coupled with moderate sequence hydrophobicity may be advantageous for conferring enhanced target selectivity for bacterial versus mammalian membranes.
中文翻译:

解偶联两亲性和疏水性:电荷聚集在阳离子抗菌肽膜相互作用中的作用
结合了高正电荷和高疏水性的肽具有高抗菌活性,如肽毒液所体现的那样,肽毒液被大自然设计为宿主膜的破坏者,但也对病原体显示出显着的功效。为了系统地研究这种现象,这里我们重点研究了松木素 W1,一种从Goeldii Pachycondyla 中分离的肽毒液ant (WLGSALKIGAKLLPSVVGLFKKKKQ) 来检查 Lys 定位是否可以广泛应用于优化现有自然序列的功能范围。我们准备了多组 ponericin W1 类似物,其中 Lys 残基要么以两亲性方式分布在整个序列 (PonAmp) 中,要么聚集在 N 端 (PonN),要么聚集在 C 端 (PonC),以及它们的对应物通过 2-4 个 Leu-to-Ala 置换降低疏水性。我们发现野生型 ponericin W1 和所有三种变体都显示出对人类红细胞的毒性,但通过用 Ala 残基替换两个或多个 Leu 残基消除了溶血。同样,含有多达 3 个 Leu-to-Ala 替代物的肽保留了对大肠杆菌的抗菌活性细菌。通过圆二色光谱对肽-膜相互作用模式的生物物理分析揭示了一种新的簇依赖肽定位模式,即 PonAmp 和 PonC 肽显示完整或部分螺旋结构,而 PonN 肽是非结构化的,部分原因可能是由于水和膜表面环境之间的动态交换。总体研究结果表明,N 端电荷簇构建体的较低膜渗透性与中等序列疏水性相结合,可能有利于增强细菌膜与哺乳动物膜的靶标选择性。
更新日期:2021-08-31
中文翻译:

解偶联两亲性和疏水性:电荷聚集在阳离子抗菌肽膜相互作用中的作用
结合了高正电荷和高疏水性的肽具有高抗菌活性,如肽毒液所体现的那样,肽毒液被大自然设计为宿主膜的破坏者,但也对病原体显示出显着的功效。为了系统地研究这种现象,这里我们重点研究了松木素 W1,一种从Goeldii Pachycondyla 中分离的肽毒液ant (WLGSALKIGAKLLPSVVGLFKKKKQ) 来检查 Lys 定位是否可以广泛应用于优化现有自然序列的功能范围。我们准备了多组 ponericin W1 类似物,其中 Lys 残基要么以两亲性方式分布在整个序列 (PonAmp) 中,要么聚集在 N 端 (PonN),要么聚集在 C 端 (PonC),以及它们的对应物通过 2-4 个 Leu-to-Ala 置换降低疏水性。我们发现野生型 ponericin W1 和所有三种变体都显示出对人类红细胞的毒性,但通过用 Ala 残基替换两个或多个 Leu 残基消除了溶血。同样,含有多达 3 个 Leu-to-Ala 替代物的肽保留了对大肠杆菌的抗菌活性细菌。通过圆二色光谱对肽-膜相互作用模式的生物物理分析揭示了一种新的簇依赖肽定位模式,即 PonAmp 和 PonC 肽显示完整或部分螺旋结构,而 PonN 肽是非结构化的,部分原因可能是由于水和膜表面环境之间的动态交换。总体研究结果表明,N 端电荷簇构建体的较低膜渗透性与中等序列疏水性相结合,可能有利于增强细菌膜与哺乳动物膜的靶标选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号