当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Copper(I) Complex with Two Unpaired Electrons, Synthesised by Oxidation of a Copper(II) Complex with Two Redox-Active Ligands
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2021-08-23 , DOI: 10.1002/anie.202109367 Marco Werr 1 , Elisabeth Kaifer 1 , Markus Enders 1 , Andika Asyuda 2 , Michael Zharnikov 2 , Hans-Jörg Himmel 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2021-08-23 , DOI: 10.1002/anie.202109367 Marco Werr 1 , Elisabeth Kaifer 1 , Markus Enders 1 , Andika Asyuda 2 , Michael Zharnikov 2 , Hans-Jörg Himmel 1
Affiliation
|
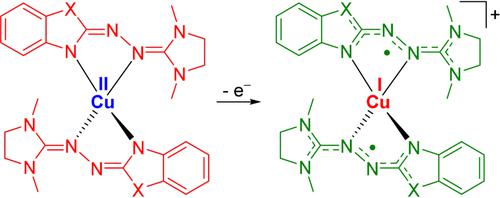
Two homoleptic copper(II) complexes [Cu(L1)2] and [Cu(L2)2] with anionic redox-active ligands were synthesised, one with urea azine (L1) and the other with thio-urea azine (L2) ligands. One-electron oxidation of the complexes initiates an unprecedented redox-induced electron transfer process, leading to monocationic copper(I) complexes [Cu(L1)2]+ and [Cu(L2)2]+ with two oxidised ligands. While [Cu(L1)2]+ is best described as a CuI complex with two neutral radical ligands that couple antiferromagnetically, [Cu(L2)2]+ is a CuI complex with two clearly different ligand units in the solid state and with a magnetic susceptibility close to a diamagnetic compound. Further one-electron oxidation of the complex with L1 ligands results in a dication [Cu(L1)2]2+, best described as a CuI complex with a twofold oxidised, monocationic ligand and a neutral radical ligand. The stability in at least three redox states, the accumulation of spin density at the ligands and the facile ligand-metal electron transfer make these complexes highly attractive for a variety of applications; here the catalytic aerobic oxidation of alcohols to aldehydes is tested.
中文翻译:

具有两个不成对电子的铜 (I) 配合物,通过氧化具有两个氧化还原活性配体的铜 (II) 配合物合成
合成了两种具有阴离子氧化还原活性配体的均配铜(II)配合物[Cu(L1) 2 ]和[Cu(L2) 2 ],一种具有脲嗪(L1),另一种具有硫脲嗪(L2)配体. 复合物的单电子氧化引发了前所未有的氧化还原诱导电子转移过程,导致单阳离子铜 (I) 复合物 [Cu(L1) 2 ] +和 [Cu(L2) 2 ] +与两个氧化配体。虽然 [Cu(L1) 2 ] +最好描述为具有两个反铁磁耦合的中性自由基配体的 Cu I 配合物,但 [Cu(L2) 2 ] +是 Cu I具有两个明显不同的固态配体单元的复合物,并且具有接近抗磁性化合物的磁化率。与 L1 配体的配合物进一步单电子氧化导致双阳离子 [Cu(L1) 2 ] 2+,最好描述为具有双重氧化的单阳离子配体和中性自由基配体的 Cu I配合物。至少三种氧化还原状态的稳定性、配体处自旋密度的积累以及配体-金属电子转移的简便性使这些配合物对各种应用极具吸引力;这里测试了醇催化有氧氧化为醛的过程。
更新日期:2021-10-12
中文翻译:

具有两个不成对电子的铜 (I) 配合物,通过氧化具有两个氧化还原活性配体的铜 (II) 配合物合成
合成了两种具有阴离子氧化还原活性配体的均配铜(II)配合物[Cu(L1) 2 ]和[Cu(L2) 2 ],一种具有脲嗪(L1),另一种具有硫脲嗪(L2)配体. 复合物的单电子氧化引发了前所未有的氧化还原诱导电子转移过程,导致单阳离子铜 (I) 复合物 [Cu(L1) 2 ] +和 [Cu(L2) 2 ] +与两个氧化配体。虽然 [Cu(L1) 2 ] +最好描述为具有两个反铁磁耦合的中性自由基配体的 Cu I 配合物,但 [Cu(L2) 2 ] +是 Cu I具有两个明显不同的固态配体单元的复合物,并且具有接近抗磁性化合物的磁化率。与 L1 配体的配合物进一步单电子氧化导致双阳离子 [Cu(L1) 2 ] 2+,最好描述为具有双重氧化的单阳离子配体和中性自由基配体的 Cu I配合物。至少三种氧化还原状态的稳定性、配体处自旋密度的积累以及配体-金属电子转移的简便性使这些配合物对各种应用极具吸引力;这里测试了醇催化有氧氧化为醛的过程。



























 京公网安备 11010802027423号
京公网安备 11010802027423号