当前位置:
X-MOL 学术
›
Drug Test. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Procollagen type III amino-terminal propeptide and insulin-like growth factor I as biomarkers of growth hormone administration
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2021-08-21 , DOI: 10.1002/dta.3155 David A Cowan 1 , Danielle A Moncrieffe 1, 2
Drug Testing and Analysis ( IF 2.6 ) Pub Date : 2021-08-21 , DOI: 10.1002/dta.3155 David A Cowan 1 , Danielle A Moncrieffe 1, 2
Affiliation
|
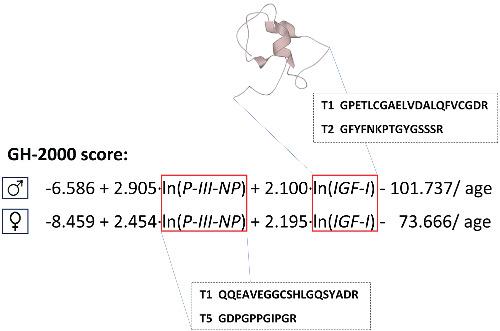
The acceptance in 2012 by the World Anti-Doping Agency (WADA) of the biomarker test for human growth hormone (hGH) based on procollagen type III amino-terminal propeptide (P-III-NP) and insulin-like growth factor I (IGF-I) was perhaps the first time that such a method has been used for forensic purposes. Developing a biomarker test to anti-doping standards, where the strict liability principle applies, is discussed. An alternative WADA-accepted approach is based on the measurement of different hGH isoforms, a method that suffers from the very short half-life of hGH limiting the detection period. Modification or withdrawal of the immunoassays, on which the biomarker measurements largely depend, has necessitated revalidation of the assays, remeasurement of samples and adjustment of the decision limits above which an athlete will be assumed to have administered hGH. When a liquid chromatography coupled mass spectrometry (LC–MS) method became a reality for the measurement of IGF-I, more consistency of results was assured. Measurement of P-III-NP is still dependent on immunoassays although work is underway to develop an LC–MS method. The promised long-term detection time for the biomarker assay does not appear to have been realised in practice, and this is perhaps partly the result of decision limits being set too high. Nevertheless, more robust assays are needed before a further adjustment of the decision limit is warranted. In the meantime, WADA is considering using P-III-NP and IGF-I as components of a biomarker passport system recording data from an individual athlete, rather than the population. Using this approach, smaller perturbations in the growth hormone (GH) score would mandate an investigation and possible action for hGH administration.
中文翻译:

前胶原 III 型氨基末端前肽和胰岛素样生长因子 I 作为生长激素给药的生物标志物
世界反兴奋剂机构 (WADA) 于 2012 年接受基于 III 型前胶原氨基末端前肽 (P-III-NP) 和胰岛素样生长因子 I (IGF) 的人类生长激素 (hGH) 生物标志物测试-I) 可能是第一次将这种方法用于法医目的。讨论了针对适用严格责任原则的反兴奋剂标准开发生物标志物测试。WADA 接受的另一种方法是基于不同 hGH 异构体的测量,这种方法的缺点是 hGH 的半衰期非常短,从而限制了检测期。生物标志物测量主要依赖于免疫测定法的修改或撤销,因此需要重新验证测定法,重新测量样本并调整运动员将被认为已施用 hGH 的决定限。当液相色谱耦合质谱 (LC-MS) 方法成为 IGF-I 测量的现实时,结果的一致性得到了保证。尽管正在开发一种 LC-MS 方法,但 P-III-NP 的测量仍然依赖于免疫测定。生物标志物分析所承诺的长期检测时间似乎并未在实践中实现,这可能部分是决策限制设置过高的结果。然而,在需要进一步调整决策限制之前,需要更强大的分析。与此同时,世界反兴奋剂机构正在考虑使用 P-III-NP 和 IGF-I 作为生物标志护照系统的组成部分,记录来自单个运动员的数据,而不是人口。使用这种方法,生长激素 (GH) 评分中较小的扰动将要求对 hGH 给药进行调查和可能的行动。
更新日期:2021-08-21
中文翻译:

前胶原 III 型氨基末端前肽和胰岛素样生长因子 I 作为生长激素给药的生物标志物
世界反兴奋剂机构 (WADA) 于 2012 年接受基于 III 型前胶原氨基末端前肽 (P-III-NP) 和胰岛素样生长因子 I (IGF) 的人类生长激素 (hGH) 生物标志物测试-I) 可能是第一次将这种方法用于法医目的。讨论了针对适用严格责任原则的反兴奋剂标准开发生物标志物测试。WADA 接受的另一种方法是基于不同 hGH 异构体的测量,这种方法的缺点是 hGH 的半衰期非常短,从而限制了检测期。生物标志物测量主要依赖于免疫测定法的修改或撤销,因此需要重新验证测定法,重新测量样本并调整运动员将被认为已施用 hGH 的决定限。当液相色谱耦合质谱 (LC-MS) 方法成为 IGF-I 测量的现实时,结果的一致性得到了保证。尽管正在开发一种 LC-MS 方法,但 P-III-NP 的测量仍然依赖于免疫测定。生物标志物分析所承诺的长期检测时间似乎并未在实践中实现,这可能部分是决策限制设置过高的结果。然而,在需要进一步调整决策限制之前,需要更强大的分析。与此同时,世界反兴奋剂机构正在考虑使用 P-III-NP 和 IGF-I 作为生物标志护照系统的组成部分,记录来自单个运动员的数据,而不是人口。使用这种方法,生长激素 (GH) 评分中较小的扰动将要求对 hGH 给药进行调查和可能的行动。











































 京公网安备 11010802027423号
京公网安备 11010802027423号