当前位置:
X-MOL 学术
›
Chem. Rec.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Supported Palladium Catalyzed Carbonylative Coupling Reactions using Carbon Monoxide as C1 Source
The Chemical Record ( IF 7.0 ) Pub Date : 2021-08-21 , DOI: 10.1002/tcr.202100157 Shaifali 1, 2 , Sheetal 1, 2 , Pralay Das 1, 2
The Chemical Record ( IF 7.0 ) Pub Date : 2021-08-21 , DOI: 10.1002/tcr.202100157 Shaifali 1, 2 , Sheetal 1, 2 , Pralay Das 1, 2
Affiliation
|
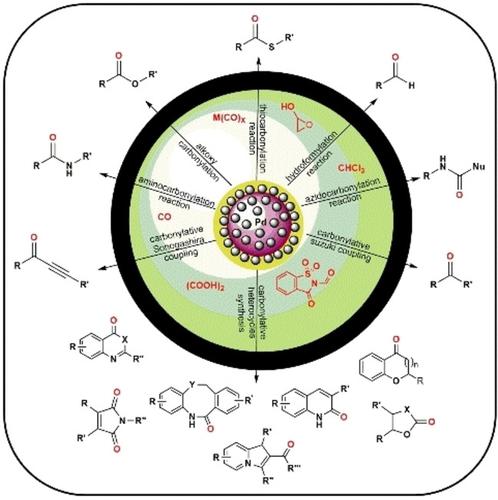
The carbonylative reactions of aryl halides, boronic acids, amines, activated alkene and alkynes under CO and supported palladium catalyzed conditions are very popular reactions for the synthesis of bioactive molecules, pharmaceuticals, polymers, peptides, intermediates and fine chemicals synthesis. Due to cost effectiveness and easy handling of recyclable supported palladium catalyst, it became more popular among researchers either working in academic institute or industry. In recent years, irrespective of poisoning effect of CO with palladium as major limitation, several advancements have been done through surface selection, designing and condition improvement to achieve high yield in the area of carbonylative coupling reactions. We hope this review will be helpful as a ready reference of last 20 years in the field of CO insertion reactions using diverse range of supported palladium catalysts under carbon monoxide or its sources as C1 source.
中文翻译:

以一氧化碳为 C1 源的负载型钯催化羰基偶联反应
芳基卤化物、硼酸、胺、活化烯烃和炔烃在 CO 和负载钯催化条件下的羰基化反应是合成生物活性分子、药物、聚合物、肽、中间体和精细化学品合成的非常流行的反应。由于可回收负载钯催化剂的成本效益和易于处理,它在学术机构或工业界的研究人员中越来越受欢迎。近年来,不考虑以钯为主要限制的 CO 中毒效应,通过表面选择、设计和条件改进,在羰基化偶联反应领域取得了高产率。
更新日期:2021-08-21
中文翻译:

以一氧化碳为 C1 源的负载型钯催化羰基偶联反应
芳基卤化物、硼酸、胺、活化烯烃和炔烃在 CO 和负载钯催化条件下的羰基化反应是合成生物活性分子、药物、聚合物、肽、中间体和精细化学品合成的非常流行的反应。由于可回收负载钯催化剂的成本效益和易于处理,它在学术机构或工业界的研究人员中越来越受欢迎。近年来,不考虑以钯为主要限制的 CO 中毒效应,通过表面选择、设计和条件改进,在羰基化偶联反应领域取得了高产率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号