Tetrahedron ( IF 2.1 ) Pub Date : 2021-08-21 , DOI: 10.1016/j.tet.2021.132411 Fumika Karaki 1, 2 , Takuto Kiguchi 1 , Kennosuke Itoh 1, 2 , Noriko Sato 3 , Kazuhide Konishi 4 , Hideaki Fujii 1, 2
|
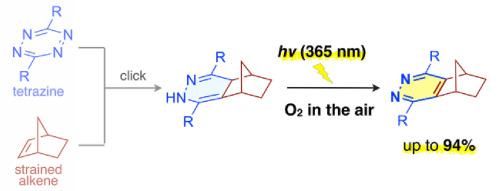
In the inverse electron-demand Diels–Alder (iEDDA) reactions between tetrazines and strained alkenes, a mixture of 1,4-dihydropyridazine isomers are formed first, and they are then oxidized to pyridazines. Although the products of these related oxidation processes converge as pyridazines, the oxidation rate is quite low with some substrates. In this study, we revealed that 1,4-dihydropyridazines formed in the iEDDA reactions were oxidized to pyridazines by simply irradiating with an ultraviolet light under an air atmosphere. Our experimental results implied that singlet oxygen was formed in the course of the reactions to oxidize the 1,4-dihydropyridazine molecules.
中文翻译:

空气中1,4-二氢哒嗪至哒嗪的无催化剂光氧化反应
在四嗪和应变烯烃之间的逆电子需求 Diels-Alder (iEDDA) 反应中,首先形成 1,4-二氢哒嗪异构体的混合物,然后将它们氧化成哒嗪。尽管这些相关氧化过程的产物会聚为哒嗪,但某些底物的氧化速率非常低。在这项研究中,我们揭示了在 iEDDA 反应中形成的 1,4-二氢哒嗪通过在空气气氛下简单地用紫外线照射而被氧化成哒嗪。我们的实验结果表明,在氧化 1,4-二氢哒嗪分子的反应过程中形成了单线态氧。











































 京公网安备 11010802027423号
京公网安备 11010802027423号