Bioelectrochemistry ( IF 5 ) Pub Date : 2021-08-21 , DOI: 10.1016/j.bioelechem.2021.107936 A Hrioua 1 , A Loudiki 2 , A Farahi 1 , F Laghrib 3 , M Bakasse 4 , S Lahrich 1 , S Saqrane 1 , M A El Mhammedi 1
|
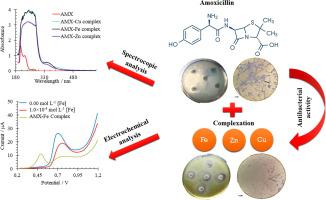
Some bacteria have developed resistance to antibiotics that were once commonly used to treat them. Moreover, this resistance has become more and more massive and worrying. During this work, we succeeded in synthesizing “metal-antibiotic” complexes, combining as a ligand for the metals of Cu (II), Zn (II) and Fe (III). These complexes AMX − M (M = Cu, Fe and Zn) were characterized by UV–Vis spectrophotometry, IR spectroscopy, and electrochemical methods. Job’s method of continuous variation suggested 1:1 metals to ligand stoichiometry for all amoxicillin complexes. The binding constant/association constant (K) of the AMX with Zn(II), Cu(II), and Fe(III) were found to be 4.46 × 104, 7.17 × 102 and 7.65 × 102 L mol−1, respectively. The IR spectra shows that the ligands coordinated to the metal ions through amino, imino, carboxylate, β-lactamic and carbonyl groups. The electrochemical results proved that amoxicillin oxidation process can be delayed by transition metal complexation. After, the complex synthesis, the antibacterial activity of ligand and its metal complexes were evaluated against Escherichia. coli bacteria by antibiogram method. The results show that the metal-amoxicillin complexes have better antibacterial activity against Escherichia coli (E. coli) than the free ligand (amoxicillin) due to the AMX protection against oxidation after complexation.
中文翻译:

过渡金属络合阿莫西林:理化和抗菌活性评价
一些细菌对曾经常用来治疗它们的抗生素产生了耐药性。而且,这种阻力越来越大,越来越令人担忧。在这项工作中,我们成功合成了“金属-抗生素”配合物,作为 Cu (II)、Zn (II) 和 Fe (III) 金属的配体。这些配合物 AMX - M (M = Cu、Fe 和 Zn) 通过紫外-可见分光光度法、红外光谱和电化学方法进行了表征。Job 的连续变化方法建议所有阿莫西林配合物的金属配体化学计量为 1:1。AMX 与 Zn(II)、Cu(II) 和 Fe(III) 的结合常数/缔合常数 (K) 分别为 4.46 × 10 4、7.17 × 10 2和 7.65 × 10 2 L mol -1, 分别。红外光谱显示配体通过氨基、亚氨基、羧酸根、β-内酰胺和羰基与金属离子配位。电化学结果证明,过渡金属络合可以延缓阿莫西林的氧化过程。之后,对配体及其金属配合物的配合物合成、抗菌活性进行了评价。用抗菌谱法测定大肠杆菌。结果表明,金属-阿莫西林复合物对大肠杆菌(E.coli)的抗菌活性优于游离配体(阿莫西林),这是由于复合后AMX对氧化的保护作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号