Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2021-08-21 , DOI: 10.1016/j.jorganchem.2021.122033 Wen-Qi Luo 1 , Xiao-Gang Du 1 , Ling-Ying Chen 1 , Chuan-Ming Jin 1
|
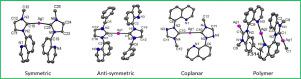
Four Ag(I)-N-heterocyclic carbene (NHC) complexes containing quinolin-8-yl groups, viz. Ag(L1)2PF6 (L1 = 1-(quinolin-8-yl)-3-methyl-1H-imidazol-2-ylidene) (7), Ag(L2)2PF6 (L2 = 1-(quinolin-8-yl)-3-benzyl-1H-imidazol-2-ylidene) (8), Ag(L3)2PF6 (L3 = 1-(quinolin-8-yl)-4-methyl-1H-1, 2, 4-triazoline-5-ylidene) (9), and Ag(L4)PF6 (L4 =1-(quinolin-8-yl)-3-methyl-1H-benzimidazolin-2-ylidene) (12), were synthesized from Ag oxide and the corresponding ligand precursors (4, 5, 6, and 11). They were characterized by proton nuclear magnetic resonance (1H NMR) spectroscopy, carbon-13 NMR (13C NMR) spectroscopy, electrospray ionization–mass spectrometry (ESI–MS), elemental analysis (EA), and single-crystal X-ray diffraction (XRD) analysis. X-ray crystallography revealed that complexes 7, 8, and 9 adopted a mononuclear Ag carbene geometry with a linear carbene C–Ag–C bond, whereas 12 exhibited a one-dimensional (1D) linear polymer with carbene C–Ag–C and quinoline N–Ag–N bonds. The structural assignments for 7 and 8 were further supported by density functional theory (DFT) calculations. Complex 9 has a distinct broad absorption at 421 nm, which could be attributed to the conjunctive effect increasing within the substituted quinoline and triazolium rings, and the strong π–π interaction between the intermolecular quinoline and triazolium rings. Study on the in vitro cytotoxic activity of 8, 9, and 12 against breast cancer MCF-7 cells indicated that complex 12 displayed the best anticancer activity in MCF-7 cells at a concentration of 5 × 10−6 mol/L. Additionally, the three Ag-NHC complexes 8, 9, and 12 had significant inhibitory effects on breast cancer cell growth at a concentration of 5 × 10−5 mol/L.
中文翻译:

四种银(I)-N-杂环卡宾配合物和一种含有喹啉-8-基的聚合物的合成、结构和抗癌活性
四种含有喹啉-8-基团的 Ag(I)-N-杂环卡宾 (NHC) 配合物,即。Ag(L 1 ) 2 PF 6 (L 1 = 1-(quinolin-8-yl)-3-methyl-1H-imidazol-2-ylidene) ( 7 ), Ag(L 2 ) 2 PF 6 (L 2 = 1-(quinolin-8-yl)-3-benzyl-1H-imidazol-2-ylidene) ( 8 ), Ag(L 3 ) 2 PF 6 (L 3 = 1-(quinolin-8-yl)-4-甲基-1H-1, 2, 4-triazoline-5-ylidene) ( 9 ) 和 Ag(L 4 )PF 6 (L 4=1-(quinolin-8-yl)-3-methyl-1H-benzimidazolin-2-ylidene) ( 12 ),由氧化银和相应的配体前体(4、5、6和11)合成。它们通过质子核磁共振 ( 1 H NMR) 光谱、碳 13 NMR ( 13 C NMR) 光谱、电喷雾电离-质谱 (ESI-MS)、元素分析 (EA) 和单晶 X 射线进行表征。衍射(XRD)分析。X 射线晶体学显示配合物7、8和9采用单核 Ag 卡宾几何结构,具有线性卡宾 C-Ag-C 键,而12展示了具有卡宾 C-Ag-C 和喹啉 N-Ag-N 键的一维(1D)线性聚合物。密度泛函理论 (DFT) 计算进一步支持了7和8的结构分配。配合物9在 421 nm 处具有明显的宽吸收,这可能归因于取代喹啉和三唑环内的联合效应增加,以及分子间喹啉和三唑环之间的强 π-π 相互作用。研究在体外的细胞毒性活性8,9,和12对乳腺癌MCF-7细胞表明复杂12在 5 × 10 -6 mol/L的浓度下,MCF-7 细胞显示出最好的抗癌活性。此外,三种Ag-NHC复合物8、9和12在5×10 -5 mol/L的浓度下对乳腺癌细胞的生长具有显着的抑制作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号