Synthesis ( IF 2.2 ) Pub Date : 2021-08-20 , DOI: 10.1055/s-0040-1719825 Daesung Lee , Sourav Ghorai
|
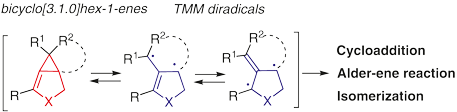
Bicyclo[3.1.0]hex-1-ene is a highly strained bicyclic intermediate that generates trimethylenemethane (TMM) diradical through breaking the C–C bond of its methylene cyclopropane moiety. The reactivity of bicyclo[3.1.0]hex-1-enes and trimethylenemethane (TMM) diradicals depend on the reaction temperature and substitution patterns. This short review covers various strain-induced transformations of bicyclo[3.1.0]hex-1-enes and their formal [3+2] cycloadditions through TMM diradicals and presents synthetic applications to natural products containing triquinane and tropone structures.
1 Introduction
2 Early Reports on Bicyclo[3.1.0]hex-1-enes
3 Approaches to Form Bicyclo[3.1.0]hex-1-enes
4 Structure and Reactivity of Bicyclo[3.1.0]hex-1-enes
4.1 Isomerization
4.2 Dimerization
4.3 [3+2] Cycloaddition
4.4 [4+2] Cycloaddition
5 Synthetic Applications to Natural Products
6 Summary and Outlook
中文翻译:

双环[3.1.0]己-1-烯的应变诱导转化
双环[3.1.0]己-1-烯是一种高度应变的双环中间体,通过破坏其亚甲基环丙烷部分的C-C键产生三亚甲基甲烷(TMM)双自由基。双环[3.1.0]己-1-烯和三亚甲基甲烷(TMM)双自由基的反应性取决于反应温度和取代模式。这篇简短的综述涵盖了双环 [3.1.0] 己-1-烯的各种应变诱导转化及其通过 TMM 双自由基的形式 [3+2] 环加成,并介绍了对含有三喹烷和托酮结构的天然产物的合成应用。
1 简介
2 双环[3.1.0]hex-1-enes 的早期报告
3 形成双环[3.1.0]己-1-烯的方法
4 双环[3.1.0]hex-1-enes的结构和反应性
4.1 异构化
4.2 二聚化
4.3 [3+2] 环加成
4.4 [4+2] 环加成
5 天然产品的合成应用
6 总结与展望











































 京公网安备 11010802027423号
京公网安备 11010802027423号