Tetrahedron ( IF 2.1 ) Pub Date : 2021-08-20 , DOI: 10.1016/j.tet.2021.132408 Ivana Kovačević 1 , Jelena Kesić 1 , Mirjana Popsavin 1 , Jovana Francuz 1 , Vesna Kojić 2 , Dimitar Jakimov 2 , Marko V. Rodić 1 , Bojana Srećo Zelenović 1 , Goran Benedeković 1 , Velimir Popsavin 1, 3
|
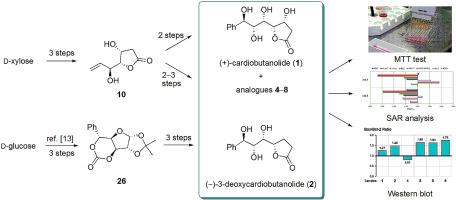
Two natural products, (+)-cardiobutanolide and (−)-3-deoxycardiobutanolide, as well as five new analogues, were synthesized in several steps that included zinc-mediated THF ring opening, subsequent stereoselective olefination, and final Sharpless asymmetric dihydroxylation. In vitro antitumour activities of these compounds were evaluated against a panel of eight human tumour cell lines and one normal cell line. Some of compounds displayed powerful effects against tumour cells, but none of them were active toward normal cells. A SAR study revealed that the change of configuration at the C-6 and C-7 position of (+)-cardiobutanolide decreases antitumour activity of analogues. It also appears that the presence of a hydroxyl group at the C-3 position increases the activity of this type of lactones. A comparison of activities of conformationally rigid lactone goniofufurone with that of more flexible cardiobutanolide and 3-deoxycardiobutanolide indicates that steric flexibility has a positive effect on cytotoxicity. It was also confirmed that removal of the phenyl group may result in analogues of higher activity. Flow cytometry analysis revealed that the synthesized compounds did not induce apoptosis and necrosis of K562 cells. However, Western blot analysis showed that all compounds but one had an increased Bax/Bcl-2 protein expression ratio.
中文翻译:

(+)-心丁内酯、(-)-3-脱氧心丁内酯和类似物作为抗增殖剂的不对称合成和生物学评价
两种天然产物(+)-心丁内酯和(-)-3-脱氧心丁内酯以及五种新的类似物通过几个步骤合成,包括锌介导的 THF 开环、随后的立体选择性烯化和最终的 Sharpless 不对称二羟基化。体外针对一组八种人类肿瘤细胞系和一种正常细胞系评估了这些化合物的抗肿瘤活性。一些化合物对肿瘤细胞显示出强大的作用,但没有一种化合物对正常细胞有活性。一项 SAR 研究表明,(+)-心丁内酯的 C-6 和 C-7 位置的构型变化会降低类似物的抗肿瘤活性。C-3 位羟基的存在似乎也增加了这类内酯的活性。构象刚性内酯 goniofufurone 的活性与更灵活的心丁内酯和 3-脱氧心丁内酯的活性的比较表明空间灵活性对细胞毒性有积极影响。还证实去除苯基可产生更高活性的类似物。流式细胞术分析显示合成的化合物不诱导K562细胞的凋亡和坏死。然而,蛋白质印迹分析表明,除一种外,所有化合物都具有增加的 Bax/Bcl-2 蛋白表达比。











































 京公网安备 11010802027423号
京公网安备 11010802027423号