Molecular Catalysis ( IF 3.9 ) Pub Date : 2021-08-20 , DOI: 10.1016/j.mcat.2021.111826 Yiming Jia 1 , Yao Nian 1 , Jinli Zhang 1 , You Han 1
|
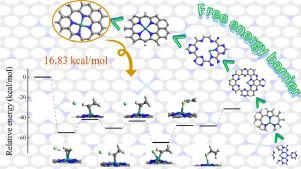
Ruthenium-based catalysts exhibit excellent catalyst activity in the acetylene hydrochlorination reaction and tend to be a good substitute for mercury-based catalysts due to their cheaper price among the precious metal catalysts. In this work, spin-polarized density functional theory (DFT) calculations were carried out to investigate the properties of the ruthenium single-atom catalysts supported on six different substrates (four pyridine nitrogen-doped carbon, four or three pyrrole nitrogen-doped carbon, C2N, CN and g-C3N4), and the reaction mechanisms of acetylene hydrochlorination catalyzed by them at 453 K. According to the results of calculations, it could be found that the reaction had the lowest free energy barrier of 16.83 kcal/mol on the Ru@4 × N6 (pyridine nitrogen-doped carbon), while it had the highest free energy barrier of 49.06 kcal/mol on the Ru@g-C3N4. This work will provide a theoretical support for the design of ruthenium single-atom catalysts for acetylene hydrochlorination reaction.
中文翻译:

不同底物的钌单原子催化剂用于乙炔氢氯化的理论设计
钌基催化剂在乙炔氢氯化反应中表现出优异的催化活性,在贵金属催化剂中价格低廉,有望成为汞基催化剂的良好替代品。在这项工作中,进行了自旋极化密度泛函理论 (DFT) 计算,以研究负载在六种不同基材(四吡啶氮掺杂碳、四或三吡咯氮掺杂碳、 C 2 N、CN 和 gC 3 N 4),以及它们在 453 K 下催化乙炔氢氯化的反应机理。根据计算结果可以发现,该反应在 Ru@4 × N6 (吡啶氮掺杂碳),而它在 Ru@gC 3 N 4上具有最高的自由能垒,为 49.06 kcal/mol 。该工作将为乙炔氢氯化反应的钌单原子催化剂的设计提供理论支持。











































 京公网安备 11010802027423号
京公网安备 11010802027423号