当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Pressure Densities and Excess Molar Volumes for the Binary Mixture of Carbon Dioxide and Hydrogen Sulfide at T = 343–397 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-19 , DOI: 10.1021/acs.jced.1c00446 Jerry A. Commodore 1 , Connor E. Deering 1 , Francis Bernard 1 , Robert A. Marriott 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-19 , DOI: 10.1021/acs.jced.1c00446 Jerry A. Commodore 1 , Connor E. Deering 1 , Francis Bernard 1 , Robert A. Marriott 1
Affiliation
|
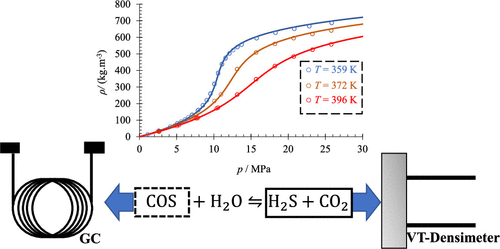
A few studies have been carried out on the volumetric properties of binary systems of H2S + CO2, which are rich in CO2; however, no volumetric studies for acid gas fluids rich in H2S have been found in the open literature. Herein we report new high-precision density measurements for three compositions (x(CO2) = 0.09822, 0.26989, and 0.49556) for the CO2 + H2S binary system at T = 343–398 K and pressures up to p = 25 MPa. The measured mixture densities were compared to data from Stouffer et al. ( J. Chem. Eng. Data 2001, 46, 1309−1318. DOI: 10.1021/je000182c.) and Nazeri et al. ( Fluid Phase Equilib. 2016, 423, 156−171. DOI: 10.1016/j.fluid.2016.04.024.The measured density data showed agreement within ca. ±2% at high pressures and up to 4% in the vicinity of the critical point or high compressibility region. Additionally, we used our mixtures and interpolated pure fluid densities to calculate the excess molar volumes, to further ascertain the accuracy of the mixing coefficients provided by Kunz and Wagner ( J. Chem. Eng. Data 2012, 57, 3032−3091. DOI: 10.1021/je300655b.). The comparison of the calculated and experimental molar volumes showed good agreement to within ±2 cm3·mol–1, especially in the high-pressure region. Within the study, we considered the COS formation from the reaction of CO2 + H2S, which was a potential explanation for measurement failure in previous studies. The measured equilibrium concentrations of COS were low and showed that the reaction of CO2 + H2S to form COS was not facile at the measured conditions.
中文翻译:

二氧化碳和硫化氢二元混合物在 T = 343–397 K 时的高压密度和过量摩尔体积
对富含CO 2的H 2 S+CO 2二元体系的体积特性进行了一些研究;然而,在公开文献中没有发现对富含 H 2 S 的酸性气体流体的体积研究。在此,我们报告了在T = 343–398 K 和压力高达p = 25 时CO 2 + H 2 S 二元系统的三种成分(x (CO 2 ) = 0.09822、0.26989 和 0.49556)的新的高精度密度测量兆帕。测量的混合物密度与来自 Stouffer 等人的数据进行了比较。( J. Chem. Eng. Data 2001 , 46 , 1309−1318 年。DOI: 10.1021/je000182c.) 和 Nazeri 等人。( Fluid Phase Equilib. 2016 , 423 , 156−171. DOI: 10.1016/j.fluid.2016.04.024. 测得的密度数据显示在高压下的一致性在大约 ±2% 内,在高压附近可达 4%临界点或高压缩率区域。此外,我们使用我们的混合物和内插纯流体密度来计算多余的摩尔体积,以进一步确定由 Kunz 和 Wagner 提供的混合系数的准确性(J. Chem. Eng. Data 2012 , 57 , 3032−3091. DOI: 10.1021/je300655b.). 计算和实验摩尔体积的比较表明在 ±2 cm 3 ·mol –1内具有良好的一致性,尤其是在高压区。在研究中,我们考虑了 CO 2 + H 2 S反应形成的 COS ,这是先前研究中测量失败的潜在解释。测量的 COS 平衡浓度较低,表明 CO 2 + H 2 S 形成 COS 的反应在测量条件下并不容易。
更新日期:2021-08-19
中文翻译:

二氧化碳和硫化氢二元混合物在 T = 343–397 K 时的高压密度和过量摩尔体积
对富含CO 2的H 2 S+CO 2二元体系的体积特性进行了一些研究;然而,在公开文献中没有发现对富含 H 2 S 的酸性气体流体的体积研究。在此,我们报告了在T = 343–398 K 和压力高达p = 25 时CO 2 + H 2 S 二元系统的三种成分(x (CO 2 ) = 0.09822、0.26989 和 0.49556)的新的高精度密度测量兆帕。测量的混合物密度与来自 Stouffer 等人的数据进行了比较。( J. Chem. Eng. Data 2001 , 46 , 1309−1318 年。DOI: 10.1021/je000182c.) 和 Nazeri 等人。( Fluid Phase Equilib. 2016 , 423 , 156−171. DOI: 10.1016/j.fluid.2016.04.024. 测得的密度数据显示在高压下的一致性在大约 ±2% 内,在高压附近可达 4%临界点或高压缩率区域。此外,我们使用我们的混合物和内插纯流体密度来计算多余的摩尔体积,以进一步确定由 Kunz 和 Wagner 提供的混合系数的准确性(J. Chem. Eng. Data 2012 , 57 , 3032−3091. DOI: 10.1021/je300655b.). 计算和实验摩尔体积的比较表明在 ±2 cm 3 ·mol –1内具有良好的一致性,尤其是在高压区。在研究中,我们考虑了 CO 2 + H 2 S反应形成的 COS ,这是先前研究中测量失败的潜在解释。测量的 COS 平衡浓度较低,表明 CO 2 + H 2 S 形成 COS 的反应在测量条件下并不容易。









































 京公网安备 11010802027423号
京公网安备 11010802027423号