当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Liquid–Liquid Equilibrium Study of the Water + Acetic Acid + Kerosene Ternary System at 293.2, 298.2, and 308.2 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-19 , DOI: 10.1021/acs.jced.1c00474 Farnaz Jafari 1 , Javad Saien 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-19 , DOI: 10.1021/acs.jced.1c00474 Farnaz Jafari 1 , Javad Saien 1
Affiliation
|
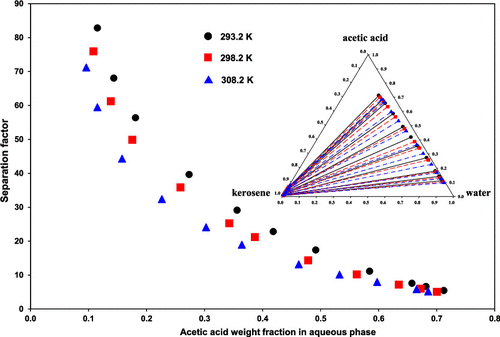
The chemical system of water + acetic acid + kerosene is frequently used in different liquid–liquid extraction studies. Accordingly, a comprehensive knowledge about this system is essential. The present study reports the capabilities of kerosene in extracting acetic acid from aqueous solutions at conventional temperatures of 293.2, 298.2, and 308.2 K and under an ambient pressure of 81.5 kPa. The cloud point method and refractive index measurement were employed in experiments. The distribution coefficient of the solute was within (0.0536–0.1161) and the important separation factor was within (5.07–82.84). The upper limit values in the ranges of the latter parameter were 1.36–8.80 times of those with other solvents. The distribution coefficient increased with temperature, but the separation factor diminished. The provided data were reproduced with the nonrandom two-liquid and universal quasichemical activity coefficient models, and the corresponding binary interactions were determined at each temperature. In this regard, a surrogate of kerosene, consisting of 80 wt % n-decane and 20 wt % 1,2,4-trimethylbenzene, was considered. The root mean square deviations of, respectively, (0.0035–0.0057) and (0.0056–0.0067) and also other criteria revealed good agreements between experimental and predicted values.
中文翻译:

水 + 乙酸 + 煤油三元系统在 293.2、298.2 和 308.2 K 下的液-液平衡研究
水 + 醋酸 + 煤油的化学体系经常用于不同的液-液萃取研究。因此,有关该系统的全面知识是必不可少的。本研究报告了煤油在 293.2、298.2 和 308.2 K 的常规温度和 81.5 kPa 的环境压力下从水溶液中提取乙酸的能力。实验采用浊点法和折光率测量法。溶质的分配系数在(0.0536-0.1161)范围内,重要的分离因子在(5.07-82.84)范围内。后者参数范围的上限值是其他溶剂的 1.36-8.80 倍。分配系数随温度升高而增加,但分离因子减小。提供的数据使用非随机的双液体和通用准化学活度系数模型进行复制,并在每个温度下确定相应的二元相互作用。在这方面,煤油的替代品,由 80 wt %考虑了正癸烷和 20 重量%的 1,2,4-三甲基苯。分别为 (0.0035–0.0057) 和 (0.0056–0.0067) 的均方根偏差以及其他标准表明实验值和预测值之间具有良好的一致性。
更新日期:2021-09-09
中文翻译:

水 + 乙酸 + 煤油三元系统在 293.2、298.2 和 308.2 K 下的液-液平衡研究
水 + 醋酸 + 煤油的化学体系经常用于不同的液-液萃取研究。因此,有关该系统的全面知识是必不可少的。本研究报告了煤油在 293.2、298.2 和 308.2 K 的常规温度和 81.5 kPa 的环境压力下从水溶液中提取乙酸的能力。实验采用浊点法和折光率测量法。溶质的分配系数在(0.0536-0.1161)范围内,重要的分离因子在(5.07-82.84)范围内。后者参数范围的上限值是其他溶剂的 1.36-8.80 倍。分配系数随温度升高而增加,但分离因子减小。提供的数据使用非随机的双液体和通用准化学活度系数模型进行复制,并在每个温度下确定相应的二元相互作用。在这方面,煤油的替代品,由 80 wt %考虑了正癸烷和 20 重量%的 1,2,4-三甲基苯。分别为 (0.0035–0.0057) 和 (0.0056–0.0067) 的均方根偏差以及其他标准表明实验值和预测值之间具有良好的一致性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号