当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Determination of Vapor Pressure, Enthalpy of Vaporization, and Heat Capacity of Methyl 4-tert-Butylbenzoate
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-18 , DOI: 10.1021/acs.jced.1c00323 Yuan Guo 1 , Linmao Tang 1 , Zuoxiang Zeng 1 , Weilan Xue 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-08-18 , DOI: 10.1021/acs.jced.1c00323 Yuan Guo 1 , Linmao Tang 1 , Zuoxiang Zeng 1 , Weilan Xue 1
Affiliation
|
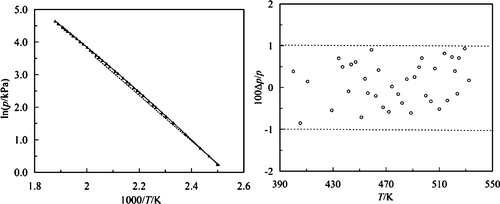
The vapor pressure of methyl 4-tert-butylbenzoate from 400.15 to 532.25 K was measured by ebulliometry and correlated by the Antoine equation with an average relative deviation (ARD) of 0.41%. The experimental results show that the atmospheric boiling point (Tb) of methyl 4-tert-butylbenzoate is 531.11 K and the molar enthalpy of vaporization at Tb is 51.24 kJ/mol according to the Clausius–Clapeyron equation. Based on Othmer’s method, using dimethyl phthalate and methyl benzoate as reference substances, the standard molar enthalpies of vaporization at 298.15 K were evaluated as 71.39 and 71.92 kJ/mol, respectively. In addition, the saturated molar heat capacity (Cs,m0) was determined by an automatic adiabatic calorimeter in the temperature range of 293.15–486.25 K and approximated by a quadratic polynomial with an ARD of 0.26%.
中文翻译:

4-叔丁基苯甲酸甲酯的蒸气压、蒸发焓和热容量的测定
4-叔丁基苯甲酸甲酯的蒸气压从 400.15 到 532.25 K 通过沸腾法测量并通过 Antoine 方程关联,平均相对偏差 (ARD) 为 0.41%。实验结果表明,根据克劳修斯-克拉珀龙方程,4-叔丁基苯甲酸甲酯的常压沸点 ( T b )为 531.11 K,T b 下的摩尔汽化焓为 51.24 kJ/mol。根据 Othmer 的方法,使用邻苯二甲酸二甲酯和苯甲酸甲酯作为参考物质,在 298.15 K 下的标准摩尔蒸发焓分别为 71.39 和 71.92 kJ/mol。此外,饱和摩尔热容 ( C s,m0) 由自动绝热量热仪在 293.15-486.25 K 的温度范围内确定,并由 ARD 为 0.26% 的二次多项式近似。
更新日期:2021-09-09
中文翻译:

4-叔丁基苯甲酸甲酯的蒸气压、蒸发焓和热容量的测定
4-叔丁基苯甲酸甲酯的蒸气压从 400.15 到 532.25 K 通过沸腾法测量并通过 Antoine 方程关联,平均相对偏差 (ARD) 为 0.41%。实验结果表明,根据克劳修斯-克拉珀龙方程,4-叔丁基苯甲酸甲酯的常压沸点 ( T b )为 531.11 K,T b 下的摩尔汽化焓为 51.24 kJ/mol。根据 Othmer 的方法,使用邻苯二甲酸二甲酯和苯甲酸甲酯作为参考物质,在 298.15 K 下的标准摩尔蒸发焓分别为 71.39 和 71.92 kJ/mol。此外,饱和摩尔热容 ( C s,m0) 由自动绝热量热仪在 293.15-486.25 K 的温度范围内确定,并由 ARD 为 0.26% 的二次多项式近似。











































 京公网安备 11010802027423号
京公网安备 11010802027423号