Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2021-08-18 , DOI: 10.1016/j.bmcl.2021.128310 Francesca Quartieri 1 , Marcella Nesi 1 , Nilla R Avanzi 1 , Daniela Borghi 1 , Elena Casale 1 , Emiliana Corti 1 , Ulisse Cucchi 1 , Daniele Donati 1 , Marina Fasolini 1 , Eduard R Felder 1 , Arturo Galvani 1 , Maria L Giorgini 1 , Antonio Lomolino 1 , Maria Menichincheri 1 , Christian Orrenius 1 , Claudia Perrera 1 , Stefania Re Depaolini 1 , Federico Riccardi-Sirtori 1 , Enea Salsi 1 , Antonella Isacchi 1 , Paola Gnocchi 1
|
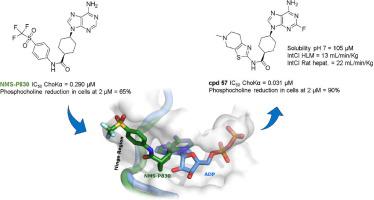
In this article we describe the identification of unprecedented ATP-competitive ChoKα inhibitors starting from initial hit NMS-P830 that binds to ChoKα in an ATP concentration-dependent manner. This result is confirmed by the co-crystal structure of NMS-P830 in complex with Δ75-ChoKα. NMS-P830 is able to inhibit ChoKα in cells resulting in the reduction of intracellular phosphocholine formation. A structure-based medicinal chemistry program resulted in the identification of selective compounds that have good biochemical activity, solubility and metabolic stability and are suitable for further optimization. The ChoKα inhibitors disclosed in this article demonstrate for the first time the possibility to inhibit ChoKα with ATP-competitive compounds.
中文翻译:

鉴定前所未有的 ATP 竞争性胆碱激酶抑制剂
在本文中,我们描述了前所未有的 ATP 竞争性 ChoKα 抑制剂的鉴定,该抑制剂从以 ATP 浓度依赖性方式与 ChoKα 结合的初始命中 NMS-P830 开始。NMS-P830 与 Δ75-ChoKα 复合的共晶结构证实了这一结果。NMS-P830 能够抑制细胞中的 ChoKα,从而减少细胞内磷酸胆碱的形成。基于结构的药物化学程序可鉴定出具有良好生化活性、溶解度和代谢稳定性并适合进一步优化的选择性化合物。本文中公开的 ChoKα 抑制剂首次证明了用 ATP 竞争性化合物抑制 ChoKα 的可能性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号