当前位置:
X-MOL 学术
›
J. Leukoc. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interference with long noncoding RNA SNHG3 alleviates cerebral ischemia-reperfusion injury by inhibiting microglial activation
Journal of Leukocyte Biology ( IF 3.6 ) Pub Date : 2021-08-19 , DOI: 10.1002/jlb.1a0421-190r Dezhang Huang 1 , Yanbin Cao 2 , Tingting Zu 3 , Jianghua Ju 4
Journal of Leukocyte Biology ( IF 3.6 ) Pub Date : 2021-08-19 , DOI: 10.1002/jlb.1a0421-190r Dezhang Huang 1 , Yanbin Cao 2 , Tingting Zu 3 , Jianghua Ju 4
Affiliation
|
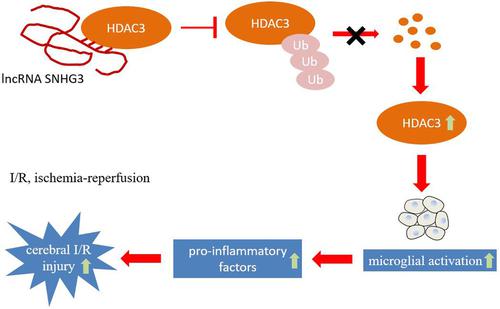
Neuroinflammation plays a strong part in cerebral ischemia-reperfusion injury, and microglial activation is regarded as a marker for neuroinflammation. Long noncoding RNA small nucleolar RNA host gene 3 (lncRNA SNHG3) is heavily expressed in cerebral ischemia-reperfusion models, but its mechanism is rarely studied. This study aims to explore whether SNHG3 is involved in cerebral ischemia-reperfusion injury by promoting microglial activation and inflammatory factor secretion. Activation of microglia was induced through oxygen-glucose deprivation/reoxygenation (OGD/R) or LPS and the cerebral ischemia-reperfusion injury in mice was induced by transient middle cerebral artery occlusion (tMCAO). Levels of SNHG3, IL-6, and TNF-α were determined by quantitative real-time PCR. Immunofluorescence was used for the detection of Iba-1 expression. Western blot was carried out for the detection of Iba-1 and histone deacetylase 3 (HDAC3) protein levels. An ELISA was performed to detect TNF-α and IL-6 levels. RNA pull-down, RNA immunoprecipitation, and co-Immunoprecipitation assays were conducted to detect the binding between SNHG3 and HDAC3. A H&E staining assay was applied to observe pathologic changes. Microglial activation was observed with immunohistochemistry. Levels of SNHG3, microglial activation marker Iba-1, proinflammatory factors (TNF-α and IL-6) were highly expressed in cell models (treated with OGD/R or LPS) and mouse models (tMCAO). Besides, SNHG3 could bind to HDAC3 and promote its expression. Through further study, we found that SNHG3 could stabilize the protein levels of HDAC3 and inhibit the ubiquitination of HDAC3. Furthermore, interference with SNHG3 down-regulated the levels of HDAC3, Iba-1, TNF-α, and IL-6, whereas the overexpression of HDAC3 reversed the results. The H&E staining assay demonstrated that the condition of vacuoles of different sizes, uneven cytoplasmic staining, and inflammatory infiltration in the brain tissue was improved by interference with SNHG3. The immunohistochemistry result showed that microglial activation marker Iba-1 was increased in the shRNA-SNHG3 group, indicating that interference with SNHG3 inhibited the activation of microglia in the brain. LncRNA SNHG3 aggravated cerebral ischemia-reperfusion injury by promoting the activation of microglia, increasing the levels of HDAC3, and the secretion of inflammatory factors.
中文翻译:

干扰长链非编码 RNA SNHG3 通过抑制小胶质细胞活化减轻脑缺血再灌注损伤
神经炎症在脑缺血再灌注损伤中起重要作用,小胶质细胞活化被认为是神经炎症的标志物。长链非编码 RNA 小核仁 RNA 宿主基因 3 (lncRNA SNHG3) 在脑缺血再灌注模型中大量表达,但其机制研究很少。本研究旨在探讨SNHG3是否通过促进小胶质细胞活化和炎症因子分泌参与脑缺血再灌注损伤。通过氧-葡萄糖剥夺/再氧合(OGD/R) 或LPS 诱导小胶质细胞的活化,并通过短暂的大脑中动脉闭塞(tMCAO) 诱导小鼠的脑缺血再灌注损伤。通过定量实时 PCR 测定 SNHG3、IL-6 和 TNF-α 的水平。免疫荧光用于检测 Iba-1 表达。进行蛋白质印迹以检测 Iba-1 和组蛋白脱乙酰酶 3 (HDAC3) 蛋白水平。进行ELISA以检测TNF-α和IL-6水平。进行 RNA 下拉、RNA 免疫沉淀和共免疫沉淀测定以检测 SNHG3 和 HDAC3 之间的结合。应用H&E染色法观察病理变化。用免疫组织化学观察小胶质细胞活化。SNHG3、小胶质细胞活化标志物 Iba-1、促炎因子(TNF-α 和 IL-6)的水平在细胞模型(用 OGD/R 或 LPS 处理)和小鼠模型(tMCAO)中高度表达。此外,SNHG3可以与HDAC3结合并促进其表达。通过进一步研究,我们发现SNHG3可以稳定HDAC3的蛋白水平并抑制HDAC3的泛素化。此外,对 SNHG3 的干扰下调了 HDAC3、Iba-1、TNF-α 和 IL-6 的水平,而 HDAC3 的过表达则逆转了结果。H&E染色结果表明,干扰SNHG3可改善脑组织中大小不一的空泡、胞质染色不均、炎症浸润等状况。免疫组化结果显示,shRNA-SNHG3组小胶质细胞活化标志物Iba-1升高,说明干扰SNHG3抑制了脑内小胶质细胞的活化。LncRNA SNHG3通过促进小胶质细胞活化、增加HDAC3水平和炎性因子分泌而加重脑缺血再灌注损伤。E染色结果表明,干扰SNHG3可改善脑组织中大小不一的空泡、胞质染色不均、炎症浸润等状况。免疫组化结果显示,shRNA-SNHG3组小胶质细胞活化标志物Iba-1升高,说明干扰SNHG3抑制了脑内小胶质细胞的活化。LncRNA SNHG3通过促进小胶质细胞活化、增加HDAC3水平和炎性因子分泌而加重脑缺血再灌注损伤。E染色结果表明,干扰SNHG3可改善脑组织中大小不一的空泡、胞质染色不均、炎症浸润等状况。免疫组化结果显示,shRNA-SNHG3组小胶质细胞活化标志物Iba-1升高,说明干扰SNHG3抑制了脑内小胶质细胞的活化。LncRNA SNHG3通过促进小胶质细胞活化、增加HDAC3水平和炎性因子分泌而加重脑缺血再灌注损伤。免疫组化结果显示,shRNA-SNHG3组小胶质细胞活化标志物Iba-1升高,说明干扰SNHG3抑制了脑内小胶质细胞的活化。LncRNA SNHG3通过促进小胶质细胞活化、增加HDAC3水平和炎性因子分泌而加重脑缺血再灌注损伤。免疫组化结果显示,shRNA-SNHG3组小胶质细胞活化标志物Iba-1升高,说明干扰SNHG3抑制了脑内小胶质细胞的活化。LncRNA SNHG3通过促进小胶质细胞活化、增加HDAC3水平和炎性因子分泌而加重脑缺血再灌注损伤。
更新日期:2021-08-19
中文翻译:

干扰长链非编码 RNA SNHG3 通过抑制小胶质细胞活化减轻脑缺血再灌注损伤
神经炎症在脑缺血再灌注损伤中起重要作用,小胶质细胞活化被认为是神经炎症的标志物。长链非编码 RNA 小核仁 RNA 宿主基因 3 (lncRNA SNHG3) 在脑缺血再灌注模型中大量表达,但其机制研究很少。本研究旨在探讨SNHG3是否通过促进小胶质细胞活化和炎症因子分泌参与脑缺血再灌注损伤。通过氧-葡萄糖剥夺/再氧合(OGD/R) 或LPS 诱导小胶质细胞的活化,并通过短暂的大脑中动脉闭塞(tMCAO) 诱导小鼠的脑缺血再灌注损伤。通过定量实时 PCR 测定 SNHG3、IL-6 和 TNF-α 的水平。免疫荧光用于检测 Iba-1 表达。进行蛋白质印迹以检测 Iba-1 和组蛋白脱乙酰酶 3 (HDAC3) 蛋白水平。进行ELISA以检测TNF-α和IL-6水平。进行 RNA 下拉、RNA 免疫沉淀和共免疫沉淀测定以检测 SNHG3 和 HDAC3 之间的结合。应用H&E染色法观察病理变化。用免疫组织化学观察小胶质细胞活化。SNHG3、小胶质细胞活化标志物 Iba-1、促炎因子(TNF-α 和 IL-6)的水平在细胞模型(用 OGD/R 或 LPS 处理)和小鼠模型(tMCAO)中高度表达。此外,SNHG3可以与HDAC3结合并促进其表达。通过进一步研究,我们发现SNHG3可以稳定HDAC3的蛋白水平并抑制HDAC3的泛素化。此外,对 SNHG3 的干扰下调了 HDAC3、Iba-1、TNF-α 和 IL-6 的水平,而 HDAC3 的过表达则逆转了结果。H&E染色结果表明,干扰SNHG3可改善脑组织中大小不一的空泡、胞质染色不均、炎症浸润等状况。免疫组化结果显示,shRNA-SNHG3组小胶质细胞活化标志物Iba-1升高,说明干扰SNHG3抑制了脑内小胶质细胞的活化。LncRNA SNHG3通过促进小胶质细胞活化、增加HDAC3水平和炎性因子分泌而加重脑缺血再灌注损伤。E染色结果表明,干扰SNHG3可改善脑组织中大小不一的空泡、胞质染色不均、炎症浸润等状况。免疫组化结果显示,shRNA-SNHG3组小胶质细胞活化标志物Iba-1升高,说明干扰SNHG3抑制了脑内小胶质细胞的活化。LncRNA SNHG3通过促进小胶质细胞活化、增加HDAC3水平和炎性因子分泌而加重脑缺血再灌注损伤。E染色结果表明,干扰SNHG3可改善脑组织中大小不一的空泡、胞质染色不均、炎症浸润等状况。免疫组化结果显示,shRNA-SNHG3组小胶质细胞活化标志物Iba-1升高,说明干扰SNHG3抑制了脑内小胶质细胞的活化。LncRNA SNHG3通过促进小胶质细胞活化、增加HDAC3水平和炎性因子分泌而加重脑缺血再灌注损伤。免疫组化结果显示,shRNA-SNHG3组小胶质细胞活化标志物Iba-1升高,说明干扰SNHG3抑制了脑内小胶质细胞的活化。LncRNA SNHG3通过促进小胶质细胞活化、增加HDAC3水平和炎性因子分泌而加重脑缺血再灌注损伤。免疫组化结果显示,shRNA-SNHG3组小胶质细胞活化标志物Iba-1升高,说明干扰SNHG3抑制了脑内小胶质细胞的活化。LncRNA SNHG3通过促进小胶质细胞活化、增加HDAC3水平和炎性因子分泌而加重脑缺血再灌注损伤。











































 京公网安备 11010802027423号
京公网安备 11010802027423号