Bioactive Materials ( IF 18.0 ) Pub Date : 2021-08-19 , DOI: 10.1016/j.bioactmat.2021.08.018 Jianrong Wu 1 , Xiaojun Cai 1 , Gareth R Williams 2 , Zheying Meng 1 , Weijuan Zou 1 , Li Yao 1 , Bing Hu 1 , Yu Chen 3 , Yuanyi Zheng 1, 4
|
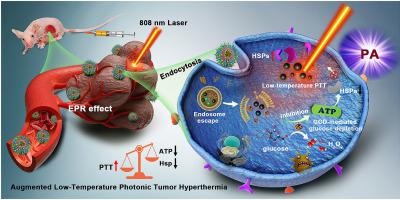
The overexpression of heat shock proteins (HSPs) in tumor cells can activate inherent defense mechanisms during hyperthermia-based treatments, inducing thermoresistance and thus diminishing the treatment efficacy. Here, we report a distinct “non-inhibitor involvement” strategy to address this issue through engineering a calcium-based nanocatalyst (G/A@CaCO3-PEG). The constructed nanocatalyst consists of calcium carbonate (CaCO3)-supported glucose oxidase (GOD) and 2D antimonene quantum dots (AQDs), with further surface modification by lipid bilayers and polyethylene glycol (PEG). The engineered G/A@CaCO3-PEG nanocatalyst features prolonged blood circulation, which is stable at neutral pH but rapidly degrades under mildly acidic tumor microenvironment, resulting in rapid release of drug cargo in the tumor microenvironment. The integrated GOD effectively catalyzes the depletion of glucose for reducing the supplies of adenosine triphosphate (ATP) and subsequent down-regulation of HSP expression. This effect then augments the therapeutic efficacy of photothermal hyperthermia induced by 2D AQDs upon irradiation with near-infrared light as assisted by reversing the cancer cells’ thermoresistance. Consequently, synergistic antineoplastic effects can be achieved via low-temperature photothermal therapy. Systematic in vitro and in vivo evaluations have demonstrated that G/A@CaCO3-PEG nanocatalysts feature potent antitumor activity with a high tumor-inhibition rate (83.92%). This work thus paves an effective way for augmenting the hyperthermia-based tumor treatments via restriction of the ATP supply.
中文翻译:

二维锑烯集成复合纳米药物通过逆转细胞耐热性增强低温光子肿瘤热疗
肿瘤细胞中热休克蛋白 (HSP) 的过度表达可以激活基于热疗的治疗过程中固有的防御机制,诱导耐热性,从而降低治疗效果。在这里,我们报告了一种独特的“非抑制剂参与”策略,通过设计钙基纳米催化剂(G/A@CaCO 3 -PEG)来解决这个问题。构建的纳米催化剂由碳酸钙 (CaCO 3 ) 支持的葡萄糖氧化酶 (GOD) 和二维锑烯量子点 (AQD) 组成,并通过脂质双层和聚乙二醇 (PEG) 进一步进行表面改性。工程化的 G/A@CaCO 3-PEG纳米催化剂具有延长血液循环的特点,在中性pH下稳定,但在弱酸性肿瘤微环境下迅速降解,导致药物在肿瘤微环境中快速释放。整合的 GOD 有效地催化葡萄糖的消耗,以减少三磷酸腺苷 (ATP) 的供应和随后的 HSP 表达下调。然后,通过逆转癌细胞的耐热性,这种作用增强了 2D AQD 在用近红外光照射后诱导的光热热疗的治疗效果。因此,可以通过低温光热疗法实现协同抗肿瘤作用。系统的体外和体内评估表明 G/A@CaCO 3-PEG纳米催化剂具有强大的抗肿瘤活性和高肿瘤抑制率(83.92%)。因此,这项工作为通过限制 ATP 供应来增强基于热疗的肿瘤治疗铺平了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号