当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
MEC17-induced α-tubulin acetylation restores mitochondrial transport function and alleviates axonal injury after intracerebral hemorrhage in mice
Journal of Neurochemistry ( IF 4.7 ) Pub Date : 2021-08-18 , DOI: 10.1111/jnc.15493 Yang Yang 1, 2, 3, 4 , Xuezhu Chen 2, 3 , Zhizhong Feng 1 , Xianfeng Cai 1 , Xiaoming Zhu 1 , Ming Cao 1 , Likun Yang 1, 4 , Yujie Chen 2, 3 , Yuhai Wang 1, 4 , Hua Feng 2, 3
Journal of Neurochemistry ( IF 4.7 ) Pub Date : 2021-08-18 , DOI: 10.1111/jnc.15493 Yang Yang 1, 2, 3, 4 , Xuezhu Chen 2, 3 , Zhizhong Feng 1 , Xianfeng Cai 1 , Xiaoming Zhu 1 , Ming Cao 1 , Likun Yang 1, 4 , Yujie Chen 2, 3 , Yuhai Wang 1, 4 , Hua Feng 2, 3
Affiliation
|
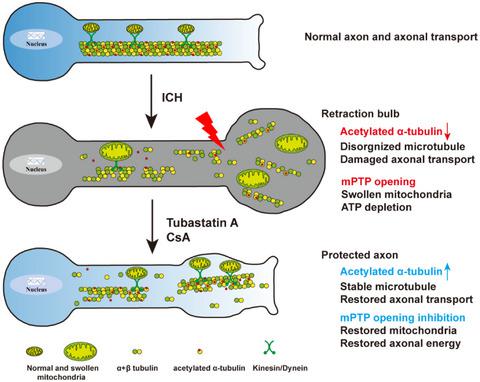
Injury to long axonal projections is a central pathological feature at the early phase of intracerebral hemorrhage (ICH). It has been reported to contribute to persistent functional disability following ICH. However, the molecular mechanisms that drive axonal degeneration remain unclear. Autologous blood was injected into the striatum to mimic the pathology of ICH. Observed significant swollen axons with characteristic retraction bulbs were found around the striatal hematoma at 24 h after ICH. Electronic microscopic examination revealed highly disorganized microtubule and swollen mitochondria in the retraction bulbs. MEC17 is a specific α-tubulin acetyltransferase, ablation of acetylated α-tubulin in MEC17−/− mice aggravated axonal injury, axonal transport mitochondria dysfunction, and motor dysfunction. In contrast, treatment with tubastatin A (TubA), which promotes microtubule acetylation, significantly alleviated axonal injury and protected the integrity of the corticospinal tract and fine motor function after ICH. Moreover, results showed that 41% mitochondria were preferentially bundled to the acetylated α-tubulin in identifiable axons and dendrites in primary neurons. This impaired axonal transport of mitochondria in primary neurons of MEC17−/− mice. Given that opening of mitochondrial permeability transition pore (mPTP) induces mitochondrial dysfunction and impairs ATP supply thereby promoting axonal injury, we enhanced the availability of acetylated α-tubulin using TubA and inhibited mPTP opening with cyclosporin A. The results indicated that this combined treatment synergistically protected corticospinal tract integrity and promoted fine motor control recovery. These findings reveal key intracellular mechanisms that drive axonal degeneration after ICH and highlight the need to target multiple factors and respective regulatory mechanisms as an effective approach to prevent axonal degeneration and motor dysfunction after ICH.
中文翻译:

MEC17 诱导的 α-微管蛋白乙酰化可恢复线粒体转运功能并减轻小鼠脑出血后的轴突损伤
长轴突投射损伤是脑出血 (ICH) 早期的中心病理特征。据报道,它会导致 ICH 后的持续性功能障碍。然而,驱动轴突变性的分子机制仍不清楚。将自体血液注入纹状体以模拟 ICH 的病理学。在 ICH 后 24 小时,在纹状体血肿周围发现了具有特征性回缩球的明显肿胀的轴突。电子显微镜检查显示回缩球中的微管高度杂乱无章,线粒体肿胀。MEC17 是一种特异性 α-微管蛋白乙酰转移酶,消融MEC17中的乙酰化 α-微管蛋白-/-小鼠加重轴突损伤、轴突转运线粒体功能障碍和运动功能障碍。相比之下,促进微管乙酰化的 tubastatin A (TubA) 治疗可显着减轻轴突损伤并保护 ICH 后皮质脊髓束的完整性和精细运动功能。此外,结果表明,41% 的线粒体优先与初级神经元中可识别的轴突和树突中的乙酰化 α-微管蛋白结合。这损害了MEC17原代神经元中线粒体的轴突运输-/-老鼠。鉴于线粒体通透性转换孔 (mPTP) 的开放会诱导线粒体功能障碍并损害 ATP 供应,从而促进轴突损伤,我们使用 TubA 增强了乙酰化 α-微管蛋白的可用性,并用环孢菌素 A 抑制了 mPTP 的开放。结果表明,这种联合治疗具有协同作用保护皮质脊髓束的完整性并促进精细运动控制的恢复。这些发现揭示了 ICH 后驱动轴突变性的关键细胞内机制,并强调需要针对多种因素和各自的调节机制作为预防 ICH 后轴突变性和运动功能障碍的有效方法。
更新日期:2021-08-18
中文翻译:

MEC17 诱导的 α-微管蛋白乙酰化可恢复线粒体转运功能并减轻小鼠脑出血后的轴突损伤
长轴突投射损伤是脑出血 (ICH) 早期的中心病理特征。据报道,它会导致 ICH 后的持续性功能障碍。然而,驱动轴突变性的分子机制仍不清楚。将自体血液注入纹状体以模拟 ICH 的病理学。在 ICH 后 24 小时,在纹状体血肿周围发现了具有特征性回缩球的明显肿胀的轴突。电子显微镜检查显示回缩球中的微管高度杂乱无章,线粒体肿胀。MEC17 是一种特异性 α-微管蛋白乙酰转移酶,消融MEC17中的乙酰化 α-微管蛋白-/-小鼠加重轴突损伤、轴突转运线粒体功能障碍和运动功能障碍。相比之下,促进微管乙酰化的 tubastatin A (TubA) 治疗可显着减轻轴突损伤并保护 ICH 后皮质脊髓束的完整性和精细运动功能。此外,结果表明,41% 的线粒体优先与初级神经元中可识别的轴突和树突中的乙酰化 α-微管蛋白结合。这损害了MEC17原代神经元中线粒体的轴突运输-/-老鼠。鉴于线粒体通透性转换孔 (mPTP) 的开放会诱导线粒体功能障碍并损害 ATP 供应,从而促进轴突损伤,我们使用 TubA 增强了乙酰化 α-微管蛋白的可用性,并用环孢菌素 A 抑制了 mPTP 的开放。结果表明,这种联合治疗具有协同作用保护皮质脊髓束的完整性并促进精细运动控制的恢复。这些发现揭示了 ICH 后驱动轴突变性的关键细胞内机制,并强调需要针对多种因素和各自的调节机制作为预防 ICH 后轴突变性和运动功能障碍的有效方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号