当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First-principles-informed energy span and microkinetic analysis of ethanol catalytic conversion to 1,3-butadiene on MgO
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2021-08-09 , DOI: 10.1039/d1cy00419k Astrid Boje 1 , William E. Taifan 2 , Henrik Ström 3 , Tomáš Bučko 4, 5 , Jonas Baltrusaitis 2 , Anders Hellman 1, 6
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2021-08-09 , DOI: 10.1039/d1cy00419k Astrid Boje 1 , William E. Taifan 2 , Henrik Ström 3 , Tomáš Bučko 4, 5 , Jonas Baltrusaitis 2 , Anders Hellman 1, 6
Affiliation
|
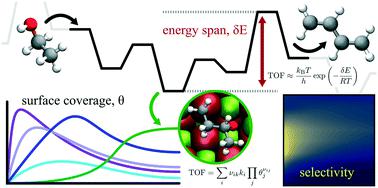
Kinetic modeling of single-step catalytic conversion of ethanol to 1,3-butadiene is necessary to inform accurate process design. This paper uses first-principles-informed energy span and microkinetic analysis to explore the reaction free energy landscapes and kinetic limitations of competing reaction pathways on a MgO (100) step-edge. Previous studies suggested mechanisms proceeding via both dehydrogenation and dehydration of ethanol, and highlighted sensitivity to conditions and catalyst composition. Here, we use the energy span concept to characterize the theoretical maximum turnover and degree of turnover frequency control for states in each reaction pathway, finding the dehydration route to be less active for 1,3-butadiene, and suggesting rate-determining states in the dehydrogenation, dehydration, and condensation steps. The influence of temperature on the relative rate contribution of each state is quantified and explained through the varying temperature sensitivity of the free energy landscape. A microkinetic model is developed to explore competition between pathways, interaction with gas-phase species, and surface coverage limitations. This suggests that the turnover may be significantly lower than predicted solely based on energetics. Turnover frequency determining states found to have high surface coverage include adsorbed ethanol and two longer, oxygenated hydrocarbons. The combined energy span and microkinetic analysis permits investigation of a complex system from two perspectives and helps elucidate conflicting observations of rate determining steps and product distribution by considering both energetic and kinetic limitations. The impact of uncertainty in the energy landscape is quantified using a correlated error model. While the range of predictions is large, the average performance and trends are similar.
中文翻译:

MgO上乙醇催化转化为1,3-丁二烯的第一性原理信息能量跨度和微动力学分析
乙醇单步催化转化为 1,3-丁二烯的动力学建模对于准确的工艺设计是必要的。本文使用第一性原理告知的能量跨度和微动力学分析来探索反应自由能景观和 MgO (100) 台阶边缘上竞争反应途径的动力学限制。以前的研究表明机制通过乙醇的脱氢和脱水,并强调对条件和催化剂组成的敏感性。在这里,我们使用能量跨度概念来表征每个反应途径中状态的理论最大周转和周转频率控制程度,发现脱水路线对 1,3-丁二烯的活性较低,并建议在脱氢、脱水和缩合步骤。温度对每个状态的相对速率贡献的影响通过自由能景观的不同温度敏感性进行量化和解释。开发了一个微动力学模型来探索途径之间的竞争、与气相物种的相互作用以及表面覆盖限制。这表明营业额可能显着低于仅基于能量学的预测。发现具有高表面覆盖率的周转频率决定状态包括吸附的乙醇和两种更长的氧化碳氢化合物。结合能量跨度和微动力学分析允许从两个角度研究复杂系统,并通过考虑能量和动力学限制帮助阐明速率确定步骤和产品分布的相互矛盾的观察结果。能源领域不确定性的影响使用相关误差模型进行量化。虽然预测范围很大,但平均性能和趋势相似。结合能量跨度和微动力学分析允许从两个角度研究复杂系统,并通过考虑能量和动力学限制帮助阐明速率确定步骤和产品分布的相互矛盾的观察结果。能源领域不确定性的影响使用相关误差模型进行量化。虽然预测范围很大,但平均性能和趋势相似。结合能量跨度和微动力学分析允许从两个角度研究复杂系统,并通过考虑能量和动力学限制帮助阐明速率确定步骤和产品分布的相互矛盾的观察结果。能源领域不确定性的影响使用相关误差模型进行量化。虽然预测范围很大,但平均性能和趋势相似。
更新日期:2021-08-19
中文翻译:

MgO上乙醇催化转化为1,3-丁二烯的第一性原理信息能量跨度和微动力学分析
乙醇单步催化转化为 1,3-丁二烯的动力学建模对于准确的工艺设计是必要的。本文使用第一性原理告知的能量跨度和微动力学分析来探索反应自由能景观和 MgO (100) 台阶边缘上竞争反应途径的动力学限制。以前的研究表明机制通过乙醇的脱氢和脱水,并强调对条件和催化剂组成的敏感性。在这里,我们使用能量跨度概念来表征每个反应途径中状态的理论最大周转和周转频率控制程度,发现脱水路线对 1,3-丁二烯的活性较低,并建议在脱氢、脱水和缩合步骤。温度对每个状态的相对速率贡献的影响通过自由能景观的不同温度敏感性进行量化和解释。开发了一个微动力学模型来探索途径之间的竞争、与气相物种的相互作用以及表面覆盖限制。这表明营业额可能显着低于仅基于能量学的预测。发现具有高表面覆盖率的周转频率决定状态包括吸附的乙醇和两种更长的氧化碳氢化合物。结合能量跨度和微动力学分析允许从两个角度研究复杂系统,并通过考虑能量和动力学限制帮助阐明速率确定步骤和产品分布的相互矛盾的观察结果。能源领域不确定性的影响使用相关误差模型进行量化。虽然预测范围很大,但平均性能和趋势相似。结合能量跨度和微动力学分析允许从两个角度研究复杂系统,并通过考虑能量和动力学限制帮助阐明速率确定步骤和产品分布的相互矛盾的观察结果。能源领域不确定性的影响使用相关误差模型进行量化。虽然预测范围很大,但平均性能和趋势相似。结合能量跨度和微动力学分析允许从两个角度研究复杂系统,并通过考虑能量和动力学限制帮助阐明速率确定步骤和产品分布的相互矛盾的观察结果。能源领域不确定性的影响使用相关误差模型进行量化。虽然预测范围很大,但平均性能和趋势相似。










































 京公网安备 11010802027423号
京公网安备 11010802027423号