当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An oxidation responsive nano-radiosensitizer increases radiotherapy efficacy by remolding tumor vasculature
Biomaterials Science ( IF 5.8 ) Pub Date : 2021-07-31 , DOI: 10.1039/d1bm00834j Xiaohui Wang 1 , Xiaoyan Niu 1 , Weizhou Sha 1 , Xiaoyue Feng 1 , Licheng Yu 1 , Zhenjie Zhang 1 , Wei Wang 1 , Zhi Yuan 1
Biomaterials Science ( IF 5.8 ) Pub Date : 2021-07-31 , DOI: 10.1039/d1bm00834j Xiaohui Wang 1 , Xiaoyan Niu 1 , Weizhou Sha 1 , Xiaoyue Feng 1 , Licheng Yu 1 , Zhenjie Zhang 1 , Wei Wang 1 , Zhi Yuan 1
Affiliation
|
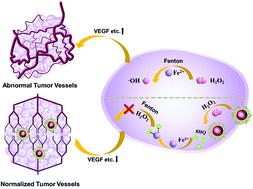
As an excellent candidate material for nano-sensitizers, gold nanostructures have shown great potential in radiotherapy. Nevertheless, severe hypoxia and low accumulation of nanomedicine caused by poor perfusion at the tumor site have significantly reduced radiotherapy efficacy. Vascular normalization has gained attention owing to its ability to relieve hypoxia and increase perfusion. The synergistic therapy of tumor vascular normalization and radiotherapy has become a new option to increase anti-cancer efficacy. However, the commonly used strategy of suppressing a single growth factor to induce vascular normalization is limited by tumor compensatory effects. In this work, we developed a strategy to inhibit oxidative stress in tumors by generating chelating agents in response to hydrogen peroxide, thereby inhibiting multi-angiogenic factors simultaneously to normalize blood vessels. Concretely, sodium alginate (SA) reacted with 8-quinoline boric acid (QBA) to form SA-QBA. Then gold nanoparticles (Au NPs) were modified with SA-QBA to obtain Au@SA-QBA. The system was simple in structure and could generate 8HQ in response to H2O2 in vitro to inhibit oxidative stress and reduce the expression of VEGF, bFGF, and Ang-2. In vivo, the perfusion unit (PU) increased by 78% after Au@SA-QBA treatment, and the coverage of pericytes increased by 32%, which in turn induced vascular normalization. In addition, blood routine and blood biochemical tests confirmed its good biocompatibility and 8HQ was not detected in the supernatant after homogenization of major organs. More importantly, after the synergistic treatment of vascular normalization and radiotherapy (4 Gy), the tumor growth inhibition rate was increased by 38.6% compared to the Au@SA-treated group with negligible side effects to normal tissues.
中文翻译:

氧化响应纳米放射增敏剂通过重塑肿瘤血管系统提高放射治疗效果
作为纳米敏化剂的优良候选材料,金纳米结构在放射治疗中显示出巨大的潜力。然而,肿瘤部位灌注不良导致的严重缺氧和纳米药物的低蓄积显着降低了放疗效果。血管正常化因其缓解缺氧和增加灌注的能力而受到关注。肿瘤血管正常化与放疗的协同治疗成为提高抗癌疗效的新选择。然而,抑制单一生长因子以诱导血管正常化的常用策略受到肿瘤代偿作用的限制。在这项工作中,我们开发了一种策略,通过产生螯合剂来响应过氧化氢来抑制肿瘤中的氧化应激,从而同时抑制多种血管生成因子,使血管正常化。具体而言,海藻酸钠 (SA) 与 8-喹啉硼酸 (QBA) 反应形成 SA-QBA。然后用SA-QBA修饰金纳米颗粒(Au NPs)以获得Au@SA-QBA。该系统结构简单,可产生8HQ响应H2 O 2 体外抑制氧化应激并降低VEGF、bFGF和Ang-2的表达。在体内,Au@SA-QBA 处理后灌注单位(PU)增加了 78%,周细胞的覆盖率增加了 32%,进而诱导血管正常化。此外,血常规和血液生化检测证实其具有良好的生物相容性,主要器官均质后上清液中未检出8HQ。更重要的是,在血管正常化和放疗(4 Gy)协同治疗后,与Au@SA治疗组相比,肿瘤生长抑制率提高了38.6%,对正常组织的副作用可忽略不计。
更新日期:2021-08-19
中文翻译:

氧化响应纳米放射增敏剂通过重塑肿瘤血管系统提高放射治疗效果
作为纳米敏化剂的优良候选材料,金纳米结构在放射治疗中显示出巨大的潜力。然而,肿瘤部位灌注不良导致的严重缺氧和纳米药物的低蓄积显着降低了放疗效果。血管正常化因其缓解缺氧和增加灌注的能力而受到关注。肿瘤血管正常化与放疗的协同治疗成为提高抗癌疗效的新选择。然而,抑制单一生长因子以诱导血管正常化的常用策略受到肿瘤代偿作用的限制。在这项工作中,我们开发了一种策略,通过产生螯合剂来响应过氧化氢来抑制肿瘤中的氧化应激,从而同时抑制多种血管生成因子,使血管正常化。具体而言,海藻酸钠 (SA) 与 8-喹啉硼酸 (QBA) 反应形成 SA-QBA。然后用SA-QBA修饰金纳米颗粒(Au NPs)以获得Au@SA-QBA。该系统结构简单,可产生8HQ响应H2 O 2 体外抑制氧化应激并降低VEGF、bFGF和Ang-2的表达。在体内,Au@SA-QBA 处理后灌注单位(PU)增加了 78%,周细胞的覆盖率增加了 32%,进而诱导血管正常化。此外,血常规和血液生化检测证实其具有良好的生物相容性,主要器官均质后上清液中未检出8HQ。更重要的是,在血管正常化和放疗(4 Gy)协同治疗后,与Au@SA治疗组相比,肿瘤生长抑制率提高了38.6%,对正常组织的副作用可忽略不计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号