当前位置:
X-MOL 学术
›
Chem. Eng. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Equilibrium Analyses and Kinetics of Carbon Dioxide Methanation Using an Alumina-Supported Ruthenium Catalyst
Chemical Engineering & Technology ( IF 1.8 ) Pub Date : 2021-08-17 , DOI: 10.1002/ceat.202100146 Duygu Uysal 1
Chemical Engineering & Technology ( IF 1.8 ) Pub Date : 2021-08-17 , DOI: 10.1002/ceat.202100146 Duygu Uysal 1
Affiliation
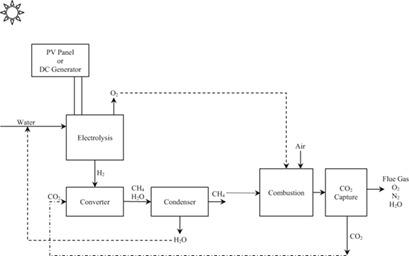
|
With CO2 being accepted as the main cause of the climate change, considerable efforts currently focus on capturing CO2 from major point sources and to store it. Another, better way is to utilize the captured CO2 to produce useful fuels like methane. The Sabatier reaction was studied in this work, using 0.5 % ruthenium on alumina as catalyst. A quartz reactor packed with catalyst particles and placed in a vertical furnace was employed. A stoichiometric ratio of CO2/H2 was used and the temperature was changed between 150 and 400 °C, while the flow rate of the feed gas mixture was varied between 250 and 625 mL min−1, with different amounts of catalyst. The temperature was identified as the major parameter affecting the conversion. Carbon dioxide conversions greater than 85 % were obtained at temperatures higher than 350 °C.
中文翻译:

使用氧化铝负载的钌催化剂进行二氧化碳甲烷化的平衡分析和动力学
随着 CO 2被认为是导致气候变化的主要原因,目前相当多的努力集中在从主要点源捕获 CO 2并将其储存起来。另一种更好的方法是利用捕获的 CO 2来生产有用的燃料,如甲烷。在这项工作中研究了 Sabatier 反应,使用氧化铝上 0.5% 的钌作为催化剂。使用装有催化剂颗粒并放置在立式炉中的石英反应器。使用 CO 2 /H 2 的化学计量比,温度在 150 至 400 °C 之间变化,同时进料气体混合物的流速在 250 至 625 mL min -1之间变化,用不同量的催化剂。温度被确定为影响转化的主要参数。在高于 350 °C 的温度下获得了大于 85% 的二氧化碳转化率。
更新日期:2021-09-20
中文翻译:

使用氧化铝负载的钌催化剂进行二氧化碳甲烷化的平衡分析和动力学
随着 CO 2被认为是导致气候变化的主要原因,目前相当多的努力集中在从主要点源捕获 CO 2并将其储存起来。另一种更好的方法是利用捕获的 CO 2来生产有用的燃料,如甲烷。在这项工作中研究了 Sabatier 反应,使用氧化铝上 0.5% 的钌作为催化剂。使用装有催化剂颗粒并放置在立式炉中的石英反应器。使用 CO 2 /H 2 的化学计量比,温度在 150 至 400 °C 之间变化,同时进料气体混合物的流速在 250 至 625 mL min -1之间变化,用不同量的催化剂。温度被确定为影响转化的主要参数。在高于 350 °C 的温度下获得了大于 85% 的二氧化碳转化率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号