Neuron ( IF 14.7 ) Pub Date : 2021-08-18 , DOI: 10.1016/j.neuron.2021.07.019 Wonchul Shin 1 , Lisi Wei 1 , Gianvito Arpino 1 , Lihao Ge 1 , Xiaoli Guo 1 , Chung Yu Chan 1 , Edaeni Hamid 1 , Oleg Shupliakov 2 , Christopher K E Bleck 3 , Ling-Gang Wu 1
|
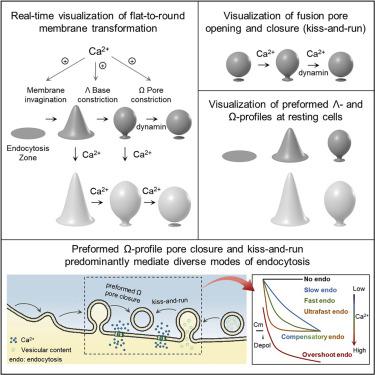
Transformation of flat membrane into round vesicles is generally thought to underlie endocytosis and produce speed-, amount-, and vesicle-size-specific endocytic modes. Visualizing depolarization-induced exocytic and endocytic membrane transformation in live neuroendocrine chromaffin cells, we found that flat membrane is transformed into Λ-shaped, Ω-shaped, and O-shaped vesicles via invagination, Λ-base constriction, and Ω-pore constriction, respectively. Surprisingly, endocytic vesicle formation is predominantly from not flat-membrane-to-round-vesicle transformation but calcium-triggered and dynamin-mediated closure of (1) Ω profiles formed before depolarization and (2) fusion pores (called kiss-and-run). Varying calcium influxes control the speed, number, and vesicle size of these pore closures, resulting in speed-specific slow (more than ∼6 s), fast (less than ∼6 s), or ultrafast (<0.6 s) endocytosis, amount-specific compensatory endocytosis (endocytosis = exocytosis) or overshoot endocytosis (endocytosis > exocytosis), and size-specific bulk endocytosis. These findings reveal major membrane transformation mechanisms underlying endocytosis, diverse endocytic modes, and exocytosis-endocytosis coupling, calling for correction of the half-a-century concept that the flat-to-round transformation predominantly mediates endocytosis after physiological stimulation.
中文翻译:

预先形成的 Ω 剖面闭合和亲吻运行介导神经内分泌嗜铬细胞中的内吞作用和多种内吞模式
平膜转化为圆形囊泡通常被认为是内吞作用的基础,并产生速度、数量和囊泡大小特定的内吞模式。观察活神经内分泌嗜铬细胞中去极化诱导的胞吐和胞吞膜转化,我们发现平膜通过内陷、Λ 基收缩和 Ω 孔收缩转化为 Λ 形、Ω 形和 O 形囊泡,分别。令人惊讶的是,内吞囊泡的形成主要不是从扁平膜到圆形囊泡的转化,而是钙触发和动力蛋白介导的 (1) 去极化前形成的 Ω 剖面和 (2) 融合孔(称为亲吻和运行) )。不同的钙流入控制着这些孔闭合的速度、数量和囊泡大小,导致速度特定的缓慢(超过~6 秒),快速(小于~6 秒)或超快(<0.6 秒)内吞作用、数量特异性补偿性内吞作用(内吞作用 = 胞吐作用)或过冲内吞作用(内吞作用 > 胞吐作用)和大小特异性的大块内吞作用。这些发现揭示了内吞作用、多种内吞模式和胞吐作用-内吞作用耦合的主要膜转化机制,呼吁纠正半个世纪以来的概念,即扁平到圆形的转化主要介导生理刺激后的内吞作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号