Chemical Engineering Research and Design ( IF 3.7 ) Pub Date : 2021-08-18 , DOI: 10.1016/j.cherd.2021.08.016 Florencia Salaberría 1, 2 , Claudio Delpino 1, 3 , Camila A. Palla 1, 2 , María E. Carrín 1, 2
|
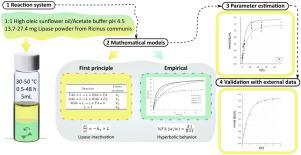
This work provides experimental data and mathematical modeling of the hydrolysis reaction of high oleic sunflower oil catalyzed by lipase powder (LP) from castor bean seeds. The production of fatty acids (FA) at different times (0.5–48 h), temperatures (30–50 °C) and concentrations of LP (0.006 and 0.012 gLP/goil) was experimentally tested. As expected, an increase in LP concentration and reaction temperature caused an increase of the initial reaction rate. The highest FA concentrations at long reaction times were obtained using 30 and 37 °C, while 45 and 50 °C showed a marked lipase thermal inactivation. Despite this, thermal inactivation effects were still detected at 37 °C using 0.006 gLP/goil, although undetected at the highest tested LP concentration. Two mathematical models were used to fit the experimental data in order to analyze FA production at tested conditions. The first one was a hyperbolic empirical model for FA concentration as a function of reaction time, which permitted the estimation of initial reaction rates and comparisons with similar reaction systems. Furthermore, a first principles model of a chain reaction system, including temperature inactivation, was also applied and kinetic constants were estimated as Arrhenius-type temperature functions. Results showed that both proposed models provided good mathematical representations of the hydrolysis reaction of high oleic sunflower oil catalyzed by LP from castor bean at the specific studied conditions. Although the second one is far more complex, it allows to predict the behavior at different conditions within the analyzed ranges, describing not only the final product (FA) but also the intermediate ones. Furthermore, this proposed first principles model was successfully validated against published experimental data. Thus, depending on the requirements and available data, both models could be helpful tools to represent and/or study the hydrolysis catalyzed by lipases from castor bean seeds.
中文翻译:

使用来自蓖麻籽粉的脂肪酶作为生物催化剂生产脂肪酸的动力学模型
这项工作提供了蓖麻籽脂肪酶粉 (LP) 催化高油酸葵花籽油水解反应的实验数据和数学模型。实验测试了不同时间(0.5-48 小时)、温度(30-50 °C)和 LP 浓度(0.006 和 0.012 g LP /g油)的脂肪酸 (FA) 产量。正如预期的那样,LP 浓度和反应温度的增加导致初始反应速率的增加。使用 30 °C和 37 °C获得了长反应时间下的最高 FA 浓度,而 45 °C和 50 °C 显示出明显的脂肪酶热失活。尽管如此,在 37 °C 下使用 0.006 g仍然检测到热灭活效应LP /g油,虽然在最高测试的 LP 浓度下未检测到。两个数学模型用于拟合实验数据,以分析测试条件下的 FA 生产。第一个是作为反应时间函数的 FA 浓度的双曲线经验模型,它允许估计初始反应速率并与类似反应系统进行比较。此外,还应用了链式反应系统的第一原理模型,包括温度失活,并将动力学常数估计为 Arrhenius 型温度函数。结果表明,在特定研究条件下,所提出的两种模型都提供了由蓖麻油 LP 催化的高油酸葵花籽油水解反应的良好数学表示。虽然第二个要复杂得多,它允许预测分析范围内不同条件下的行为,不仅描述最终产品 (FA),还描述中间产品。此外,该提议的第一原理模型已根据已发布的实验数据成功验证。因此,根据要求和可用数据,这两种模型都可以成为代表和/或研究蓖麻籽脂肪酶催化水解的有用工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号