Chem ( IF 19.1 ) Pub Date : 2021-08-18 , DOI: 10.1016/j.chempr.2021.07.013 Guang-Jian Mei 1, 2 , Jonathan J. Wong 3 , Wenrui Zheng 2 , Anjanay A. Nangia 3 , K.N. Houk 3 , Yixin Lu 2, 4
|
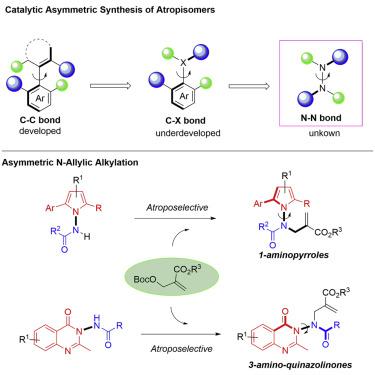
The first catalytic asymmetric synthesis of N–N axially chiral compounds has been accomplished via a quinidine catalyzed N-allylic alkylation reaction. These N–N axially chiral frameworks are a new addition to the families of axially chiral molecules and to the atropisomerism involving heteroatom(s), e.g., N, O, and S. The reaction takes place smoothly under mild conditions and displays excellent functional group tolerance, allowing facile access to a variety of N–N axially chiral 1-aminopyrroles and 3-aminoquinazolinones in high yields and excellent enantioselectivities. DFT calculations have been applied to understand the origin of enantioselectivity and provide guidance for the design of additional molecules of this type. The investigation of N–N axis atropisomerism holds promise for new discoveries in medicinal chemistry and asymmetric catalysis.
中文翻译:

N-N轴向手性化合物的合理设计和阻变选择性合成
N-N 轴向手性化合物的第一个催化不对称合成是通过奎尼丁催化的 N-烯丙基烷基化反应完成的。这些 N-N 轴向手性骨架是轴向手性分子家族和涉及杂原子(例如 N、O 和 S)的阻转异构现象的新成员。反应在温和条件下顺利进行,并显示出优异的官能团耐受性,允许以高产率和优异的对映选择性轻松获得各种 N-N 轴向手性 1-氨基吡咯和 3-氨基喹唑啉酮。DFT 计算已用于了解对映选择性的起源,并为设计此类其他分子提供指导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号