当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coordination Chemistry and Sensing Properties Towards Anions and Metal Ions of a Simple Fluorescent Urea
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2021-08-17 , DOI: 10.1002/ejic.202100580 M. Carla Aragoni 1 , Massimiliano Arca 2 , Simon J. Coles 3 , Vito Lippolis 2 , Jessica Milia 4 , James B. Orton 3 , Laura Pala 2 , Giacomo Picci 2 , Tiziana Pivetta 4 , Claudia Caltagirone 5 , Riccardo Montis 6
European Journal of Inorganic Chemistry ( IF 2.3 ) Pub Date : 2021-08-17 , DOI: 10.1002/ejic.202100580 M. Carla Aragoni 1 , Massimiliano Arca 2 , Simon J. Coles 3 , Vito Lippolis 2 , Jessica Milia 4 , James B. Orton 3 , Laura Pala 2 , Giacomo Picci 2 , Tiziana Pivetta 4 , Claudia Caltagirone 5 , Riccardo Montis 6
Affiliation
|
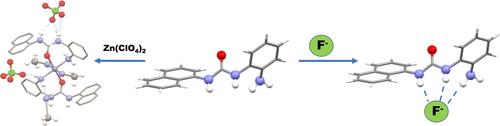
The coordination and sensing properties towards anions and transition metal ions of the simple novel fluorescent urea 1-(2-aminophenyl)-3-(naphthalen-1)-yl)urea (L) were investigated in solution, and in the solid state. An electron donating amine group in the molecular skeleton of L decreased the acidity of the urea NHs that are usually deprotonated by basic anions and allowed for a good degree of affinity towards fluoride in DMSO-d6-0.5 %H2O. Moreover, the amine moiety acted as a further binding group for metal ions. Indeed, L was able to bind Zn2+ both in solution and in the solid state, and to respond to the presence of this metal ion in MeCN with an enhancement of the fluorescence emission. Although solution studies evidenced the formation of a 1 : 1 complex of L with Zn2+, complexes with a 2 : 1 ligand-to-metal stoichiometry were isolated in the solid state. DFT calculations helped to clarify the stability reasons behind these results.
中文翻译:

简单荧光尿素对阴离子和金属离子的配位化学和传感特性
在溶液和固态中研究了简单的新型荧光尿素 1-(2-氨基苯基)-3-(萘-1)-基)脲 ( L )对阴离子和过渡金属离子的配位和传感特性。L分子骨架中的给电子胺基团降低了通常被碱性阴离子去质子化的尿素 NH 的酸度,并允许对 DMSO- d 6 -0.5 %H 2 O 中的氟化物具有良好的亲和力。此外,胺部分充当金属离子的进一步结合基团。事实上,L能够结合 Zn 2+以溶液和固态两种状态,并通过增强荧光发射来响应 MeCN 中该金属离子的存在。尽管溶液研究证明形成了L与 Zn 2+的 1:1配合物,但在固态下分离出配体与金属化学计量比为 2:1 的配合物。DFT 计算有助于阐明这些结果背后的稳定性原因。
更新日期:2021-10-01
中文翻译:

简单荧光尿素对阴离子和金属离子的配位化学和传感特性
在溶液和固态中研究了简单的新型荧光尿素 1-(2-氨基苯基)-3-(萘-1)-基)脲 ( L )对阴离子和过渡金属离子的配位和传感特性。L分子骨架中的给电子胺基团降低了通常被碱性阴离子去质子化的尿素 NH 的酸度,并允许对 DMSO- d 6 -0.5 %H 2 O 中的氟化物具有良好的亲和力。此外,胺部分充当金属离子的进一步结合基团。事实上,L能够结合 Zn 2+以溶液和固态两种状态,并通过增强荧光发射来响应 MeCN 中该金属离子的存在。尽管溶液研究证明形成了L与 Zn 2+的 1:1配合物,但在固态下分离出配体与金属化学计量比为 2:1 的配合物。DFT 计算有助于阐明这些结果背后的稳定性原因。



























 京公网安备 11010802027423号
京公网安备 11010802027423号