Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2021-08-19 , DOI: 10.1016/j.addr.2021.113940 Simon Staubach 1 , Fabiola Nardi Bauer 1 , Tobias Tertel 1 , Verena Börger 1 , Oumaima Stambouli 1 , Denise Salzig 2 , Bernd Giebel 1
|
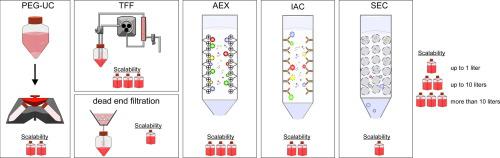
Extracellular vesicles (EVs) especially of mesenchymal stem/stomal cells (MSCs) are increasingly considered as biotherapeutic agents for a variety of different diseases. For translating them effectively into the clinics, scalable production processes fulfilling good manufacturing practice (GMP) are needed. Like for other biotherapeutic agents, the manufacturing of EV products can be subdivided in the upstream and downstream processing and the subsequent quality control, each of them containing several unit operations. During upstream processing (USP), cells are isolated, stored (cell banking) and expanded; furthermore, EV-containing conditioned media are produced. During downstream processing (DSP), conditioned media (CM) are processed to obtain concentrated and purified EV products. CM are either stored until DSP or are directly processed. As first unit operation in DSP, clarification removes remaining cells, debris and other larger impurities. The key operations of each EV DSP is volume-reduction combined with purification of the concentrated EVs. Most of the EV preparation methods used in conventional research labs including differential centrifugation procedures are limited in their scalability. Consequently, it is a major challenge in the therapeutic EV field to identify appropriate EV concentration and purification methods allowing scale up. As EVs share several features with enveloped viruses, that are used for more than two decades in the clinics now, several principles can be adopted to EV manufacturing. Here, we introduce and discuss volume reducing and purification methods frequently used for viruses and analyze their value for the manufacturing of EV-based therapeutics.
中文翻译:

从条件培养基中大规模制备细胞外囊泡
细胞外囊泡 (EVs) 尤其是间充质干细胞/造口细胞 (MSCs) 越来越多地被视为各种不同疾病的生物治疗剂。为了将它们有效地转化为临床,需要符合良好生产规范 (GMP) 的可扩展生产流程。与其他生物治疗药物一样,EV产品的制造可以细分为上下游加工和后续的质量控制,每个都包含几个单元操作。在上游处理 (USP) 期间,细胞被分离、储存(细胞库)和扩增;此外,生产含有 EV 的条件培养基。在下游加工 (DSP) 期间,对条件培养基 (CM) 进行加工以获得浓缩和纯化的 EV 产品。CM 要么被存储到 DSP,要么被直接处理。作为 DSP 中的第一个单元操作,澄清会去除剩余的细胞、碎片和其他较大的杂质。每个 EV DSP 的关键操作是体积减小与浓缩 EV 的净化相结合。传统研究实验室中使用的大多数 EV 制备方法,包括差速离心程序,其可扩展性有限。因此,确定合适的 EV 浓度和纯化方法以扩大规模是治疗性 EV 领域的一项重大挑战。由于 EV 与包膜病毒有几个共同的特征,现在已经在临床中使用了 20 多年,因此可以在 EV 制造中采用一些原则。在这里,我们介绍和讨论了病毒常用的减容和纯化方法,并分析了它们在制造基于 EV 的疗法中的价值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号