当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fast and Selective Reaction of 2-Benzylacrylaldehyde with 1,2-Aminothiol for Stable N-Terminal Cysteine Modification and Peptide Cyclization
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2021-08-18 , DOI: 10.1021/acs.bioconjchem.1c00378 Yaqi Wu 1 , Cong Li 1 , Shihui Fan 1 , Yibing Zhao 1 , Chuanliu Wu 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2021-08-18 , DOI: 10.1021/acs.bioconjchem.1c00378 Yaqi Wu 1 , Cong Li 1 , Shihui Fan 1 , Yibing Zhao 1 , Chuanliu Wu 1
Affiliation
|
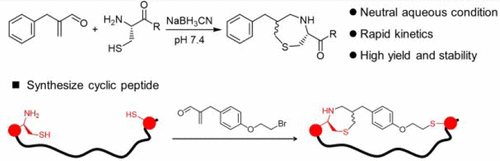
N-terminal cysteine (Cys)-specific reactions have been exploited for protein and peptide modifications. However, existing reactions for N-terminal Cys suffer from low reaction rate, unavoidable side reactions, or poor stability for reagents or products. Herein we report a fast, efficient, and selective conjugation between 2-benzylacrylaldehyde (BAA) and 1,2-aminothiol, which involves multistep reactions including aldimine condensation, Michael addition, and reduction of imine by NaBH3CN. This conjugation proceeds with a rate constant of ∼2700 M–1 s–1 under neutral condition at room temperature to produce a pair of seven-membered ring diastereoisomers, which are stable under neutral and acidic conditions. This method enables the selective modifications of the N-terminal Cys residue without interference from the internal Cys and lysine residues, providing a useful alternative to existing approaches for site-specific peptide or protein modifications and synthesis of cyclic peptides.
中文翻译:

2-苄基丙烯醛与 1,2-氨基硫醇的快速选择性反应可实现稳定的 N 端半胱氨酸修饰和肽环化
N 末端半胱氨酸 (Cys) 特异性反应已用于蛋白质和肽修饰。然而,现有的N端Cys反应存在反应速率低、副反应不可避免、试剂或产物稳定性差等问题。在此,我们报道了2-苄基丙烯醛(BAA)和1,2-氨基硫醇之间的快速、高效和选择性缀合,该缀合涉及多步反应,包括醛亚胺缩合、迈克尔加成和NaBH 3 CN还原亚胺。这种共轭反应在室温中性条件下以∼2700 M –1 s –1的速率常数进行,产生一对七元环非对映异构体,它们在中性和酸性条件下稳定。该方法能够选择性地修饰 N 端 Cys 残基,而不受内部 Cys 和赖氨酸残基的干扰,为现有的位点特异性肽或蛋白质修饰和环肽合成方法提供了一种有用的替代方法。
更新日期:2021-09-15
中文翻译:

2-苄基丙烯醛与 1,2-氨基硫醇的快速选择性反应可实现稳定的 N 端半胱氨酸修饰和肽环化
N 末端半胱氨酸 (Cys) 特异性反应已用于蛋白质和肽修饰。然而,现有的N端Cys反应存在反应速率低、副反应不可避免、试剂或产物稳定性差等问题。在此,我们报道了2-苄基丙烯醛(BAA)和1,2-氨基硫醇之间的快速、高效和选择性缀合,该缀合涉及多步反应,包括醛亚胺缩合、迈克尔加成和NaBH 3 CN还原亚胺。这种共轭反应在室温中性条件下以∼2700 M –1 s –1的速率常数进行,产生一对七元环非对映异构体,它们在中性和酸性条件下稳定。该方法能够选择性地修饰 N 端 Cys 残基,而不受内部 Cys 和赖氨酸残基的干扰,为现有的位点特异性肽或蛋白质修饰和环肽合成方法提供了一种有用的替代方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号