Cell Calcium ( IF 4 ) Pub Date : 2021-08-17 , DOI: 10.1016/j.ceca.2021.102457 Katarzyna Robaszkiewicz 1 , Ewelina Jurewicz 2 , Joanna Moraczewska 1 , Anna Filipek 2
|
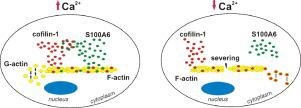
S100A6 is a Ca2+-binding protein belonging to the S100 family. Many reports indicate that S100A6 is involved in actin filament organization, however the mechanism of S100A6 action in this process is not fully understood. By screening S100A6 binding partners in NIH3T3 mouse fibroblasts, we have found that S100A6 binds cofilin-1, a protein required for the dynamics of actin polymerization and depolymerization. By applying various biochemical and cell biology assays, we have shown that S100A6 bound to cofilin-1 in a Ca2+-dependent manner and increased cofilin-1 affinity for F-actin. Microscopic analysis indicated that S100A6 significantly decreased severing of the actin filaments induced by cofilin-1. Moreover, in the presence of cofilin-1, S100A6 stabilized the filaments by inhibiting their depolymerization. When S100A6 was present at sub-stoichiometric concentrations in relation to actin, polymerization of G-actin accelerated by cofilin-1 was increased. At higher S100A6:actin ratios the polymerization rate was decreased. Altogether, these results show that S100A6 regulates actin filament dynamics by controlling activity of cofilin-1 and suggest that this regulation is Ca2+ -dependent.
中文翻译:

S100A6与cofilin-1的Ca2+依赖性结合调节肌动蛋白丝聚合-解聚动力学
S100A6 是属于S100 家族的Ca 2+结合蛋白。许多报道表明S100A6参与肌动蛋白丝的组织,但S100A6在此过程中的作用机制尚不完全清楚。通过筛选 NIH3T3 小鼠成纤维细胞中的 S100A6 结合伙伴,我们发现 S100A6 结合 cofilin-1,这是一种肌动蛋白聚合和解聚动力学所需的蛋白质。通过应用各种生化和细胞生物学测定,我们表明 S100A6 在 Ca 2+ 中与 cofilin-1 结合依赖方式和增加的 cofilin-1 对 F-肌动蛋白的亲和力。显微镜分析表明,S100A6 显着降低了由 cofilin-1 诱导的肌动蛋白丝的切断。此外,在 cofilin-1 的存在下,S100A6 通过抑制它们的解聚来稳定细丝。当 S100A6 以相对于肌动蛋白的亚化学计量浓度存在时,cofilin-1 加速的 G-肌动蛋白聚合增加。在较高的 S100A6:肌动蛋白比率下,聚合速率降低。总之,这些结果表明 S100A6 通过控制 cofilin-1 的活性来调节肌动蛋白丝的动力学,并表明这种调节是 Ca 2+依赖性的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号