Molecular Cell ( IF 14.5 ) Pub Date : 2021-08-17 , DOI: 10.1016/j.molcel.2021.08.007 Adam Osinski 1 , Miles H Black 1 , Krzysztof Pawłowski 2 , Zhe Chen 3 , Yang Li 3 , Vincent S Tagliabracci 4
|
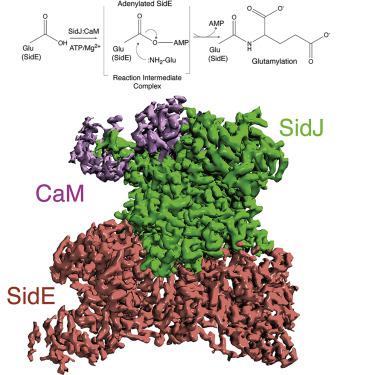
The kinase domain transfers phosphate from ATP to substrates. However, the Legionella effector SidJ adopts a kinase fold, yet catalyzes calmodulin (CaM)-dependent glutamylation to inactivate the SidE ubiquitin ligases. The structural and mechanistic basis in which the kinase domain catalyzes protein glutamylation is unknown. Here we present cryo-EM reconstructions of SidJ:CaM:SidE reaction intermediate complexes. We show that the kinase-like active site of SidJ adenylates an active-site Glu in SidE, resulting in the formation of a stable reaction intermediate complex. An insertion in the catalytic loop of the kinase domain positions the donor Glu near the acyl-adenylate for peptide bond formation. Our structural analysis led us to discover that the SidJ paralog SdjA is a glutamylase that differentially regulates the SidE ligases during Legionella infection. Our results uncover the structural and mechanistic basis in which the kinase fold catalyzes non-ribosomal amino acid ligations and reveal an unappreciated level of SidE-family regulation.
中文翻译:

激酶折叠蛋白谷氨酰化的结构和机制基础
激酶结构域将磷酸盐从 ATP 转移到底物。然而,军团菌效应器 SidJ 采用激酶折叠,但催化钙调蛋白 (CaM) 依赖性谷氨酰化以灭活 SidE 泛素连接酶。激酶结构域催化蛋白质谷氨酰化的结构和机制基础尚不清楚。在这里,我们展示了 SidJ:CaM:SidE 反应中间配合物的低温 EM 重建。我们表明 SidJ 的激酶样活性位点使 SidE 中的活性位点 Glu 腺苷酸化,从而形成稳定的反应中间复合物。在激酶结构域的催化环中的插入将供体 Glu 定位在酰基腺苷酸附近以形成肽键。我们的结构分析使我们发现 SidJ paralog SdjA 是一种谷氨酰胺酶,它在军团菌期间差异调节 SidE 连接酶感染。我们的研究结果揭示了激酶折叠催化非核糖体氨基酸连接的结构和机制基础,并揭示了一个未被认可的 SidE 家族调控水平。











































 京公网安备 11010802027423号
京公网安备 11010802027423号