当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and Evaluation of New Quinazolin-4(3H)-one Derived PqsR Antagonists as Quorum Sensing Quenchers in Pseudomonas aeruginosa
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-08-17 , DOI: 10.1021/acsinfecdis.1c00175 Fadi Soukarieh 1, 2 , Alaa Mashabi 3 , William Richardson 3 , Eduard Vico Oton 1 , Manuel Romero 1, 2 , Shaun N Roberston 1, 2 , Scott Grossman 3 , Tomas Sou 4 , Ruiling Liu 3 , Nigel Halliday 1 , Irena Kukavica-Ibrulj 5 , Roger C Levesque 5 , Christel A S Bergstrom 4 , Barrie Kellam 3 , Jonas Emsley 2, 3 , Stephan Heeb 1 , Paul Williams 1, 2 , Michael J Stocks 2, 3 , Miguel Cámara 1, 2
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2021-08-17 , DOI: 10.1021/acsinfecdis.1c00175 Fadi Soukarieh 1, 2 , Alaa Mashabi 3 , William Richardson 3 , Eduard Vico Oton 1 , Manuel Romero 1, 2 , Shaun N Roberston 1, 2 , Scott Grossman 3 , Tomas Sou 4 , Ruiling Liu 3 , Nigel Halliday 1 , Irena Kukavica-Ibrulj 5 , Roger C Levesque 5 , Christel A S Bergstrom 4 , Barrie Kellam 3 , Jonas Emsley 2, 3 , Stephan Heeb 1 , Paul Williams 1, 2 , Michael J Stocks 2, 3 , Miguel Cámara 1, 2
Affiliation
|
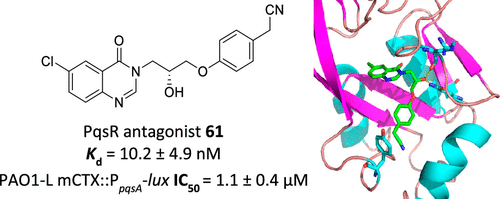
P. aeruginosa (PA) continues to pose a threat to global public health due to its high levels of antimicrobial resistance (AMR). The ongoing AMR crisis has led to an alarming shortage of effective treatments for resistant microbes, and hence there is a pressing demand for the development of novel antimicrobial interventions. The potential use of antivirulence therapeutics to tackle bacterial infections has attracted considerable attention over the past decades as they hamper the pathogenicity of target microbes with reduced selective pressure, minimizing the emergence of resistance. One such approach is to interfere with the PA pqs quorum sensing system which upon the interaction of PqsR, a Lys-R type transcriptional regulator, with its cognate signal molecules 4-hydroxy-2-heptylquinoline (HHQ) and 2-heptyl-3-hydroxy-4-quinolone (PQS), governs multiple virulence traits and host–microbe interactions. In this study, we report the hit identification and optimization of PqsR antagonists using virtual screening coupled with whole cell assay validation. The optimized hit compound 61 ((R)-2-(4-(3-(6-chloro-4-oxoquinazolin-3(4H)-yl)-2-hydroxypropoxy)phenyl)acetonitrile) was found to inhibit the expression of the PA PpqsA promoter controlled by PqsR with an IC50 of 1 μM. Using isothermal titration calorimetry, a Kd of 10 nM for the PqsR ligand binding domain (PqsRLBD) was determined for 61. Furthermore, the crystal structure of 61 with PqsRLBD was attained with a resolution of 2.65 Å. Compound 61 significantly reduced levels of pyocyanin, PQS, and HHQ in PAO1-L, PA14 lab strains and PAK6085 clinical isolate. Furthermore, this compound potentiated the effect of ciprofloxacin in early stages of biofilm treatment and in Galleria mellonella infected with PA. Altogether, this data shows 61 as a potent PqsR inhibitor with potential for hit to lead optimization toward the identification of a PA QS inhibitor which can be advanced into preclinical development.
中文翻译:

新型 Quinazolin-4(3H)-one 衍生的 PqsR 拮抗剂作为铜绿假单胞菌群体感应淬灭剂的设计和评价
铜绿假单胞菌(PA) 由于其高水平的抗菌素耐药性 (AMR),继续对全球公共卫生构成威胁。持续的 AMR 危机导致对耐药微生物有效治疗的严重短缺,因此迫切需要开发新型抗菌干预措施。在过去的几十年中,抗病毒疗法在解决细菌感染方面的潜在用途引起了相当多的关注,因为它们通过降低选择压力来阻碍目标微生物的致病性,从而最大限度地减少耐药性的出现。一种这样的方法是干扰 PA pqs群体感应系统,在 PqsR(一种 Lys-R 型转录调节因子)与其同源信号分子 4-羟基-2-庚基喹啉 (HHQ) 和 2-庚基-3-羟基-4-喹诺酮 (PQS) 相互作用时,控制多重毒力特征和宿主-微生物相互作用。在这项研究中,我们报告了使用虚拟筛选与全细胞测定验证相结合的 PqsR 拮抗剂的命中识别和优化。发现优化的命中化合物61 (( R )-2-(4-(3-(6-chloro-4-oxoquinazolin-3(4 H )-yl)-2-hydroxypropoxy)phenyl)乙腈) 抑制表达由 PqsR 控制的 PA P pqsA启动子的 IC 50为 1 μM。使用等温滴定量热法,K d61的 PqsR 配体结合域 (PqsR LBD ) 为10 nM 。此外,具有 PqsR LBD的61的晶体结构以 2.65 Å 的分辨率获得。化合物61显着降低了 PAO1-L、PA14 实验室菌株和 PAK6085 临床分离株中绿脓素、PQS 和 HHQ 的水平。此外,这种化合物增强了环丙沙星在生物膜治疗的早期阶段和被 PA 感染的大蜡螟的作用。总而言之,该数据显示61是一种有效的 PqsR 抑制剂,具有潜在的命中率,可以优化 PA QS 抑制剂的鉴定,该抑制剂可以推进临床前开发。
更新日期:2021-09-10
中文翻译:

新型 Quinazolin-4(3H)-one 衍生的 PqsR 拮抗剂作为铜绿假单胞菌群体感应淬灭剂的设计和评价
铜绿假单胞菌(PA) 由于其高水平的抗菌素耐药性 (AMR),继续对全球公共卫生构成威胁。持续的 AMR 危机导致对耐药微生物有效治疗的严重短缺,因此迫切需要开发新型抗菌干预措施。在过去的几十年中,抗病毒疗法在解决细菌感染方面的潜在用途引起了相当多的关注,因为它们通过降低选择压力来阻碍目标微生物的致病性,从而最大限度地减少耐药性的出现。一种这样的方法是干扰 PA pqs群体感应系统,在 PqsR(一种 Lys-R 型转录调节因子)与其同源信号分子 4-羟基-2-庚基喹啉 (HHQ) 和 2-庚基-3-羟基-4-喹诺酮 (PQS) 相互作用时,控制多重毒力特征和宿主-微生物相互作用。在这项研究中,我们报告了使用虚拟筛选与全细胞测定验证相结合的 PqsR 拮抗剂的命中识别和优化。发现优化的命中化合物61 (( R )-2-(4-(3-(6-chloro-4-oxoquinazolin-3(4 H )-yl)-2-hydroxypropoxy)phenyl)乙腈) 抑制表达由 PqsR 控制的 PA P pqsA启动子的 IC 50为 1 μM。使用等温滴定量热法,K d61的 PqsR 配体结合域 (PqsR LBD ) 为10 nM 。此外,具有 PqsR LBD的61的晶体结构以 2.65 Å 的分辨率获得。化合物61显着降低了 PAO1-L、PA14 实验室菌株和 PAK6085 临床分离株中绿脓素、PQS 和 HHQ 的水平。此外,这种化合物增强了环丙沙星在生物膜治疗的早期阶段和被 PA 感染的大蜡螟的作用。总而言之,该数据显示61是一种有效的 PqsR 抑制剂,具有潜在的命中率,可以优化 PA QS 抑制剂的鉴定,该抑制剂可以推进临床前开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号